Higher Blood Uric Acid in Female Humans and Mice as a Protective Factor against Pathophysiological Decline of Lung Function
Abstract
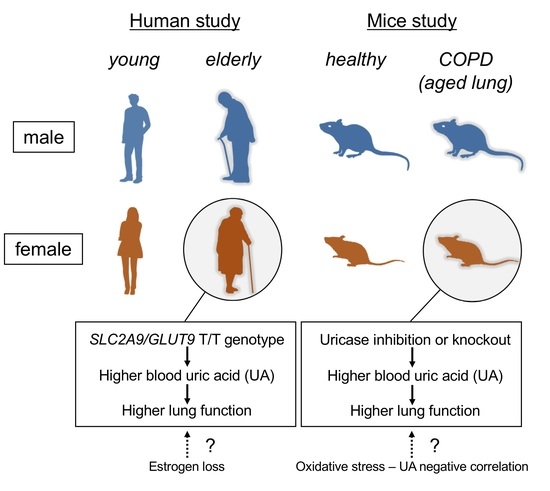
:1. Introduction
2. Materials and Methods
2.1. Animal Sources and Care
2.2. Oxonate Treatment
2.3. Elastase-Induced Pulmonary Emphysema Model
2.4. Measurement of Pulmonary Mechanics and Function by flexiVent
2.5. Histological Analysis and the Measurement of Mean Linear Intercepts (MLI)
2.6. Blood Collection and Measurement of Plasma UA and BUN
2.7. Evaluation of Plasma Antioxidant Capacity and Oxidative Stress Level
2.8. Cell Culture
2.9. Intracellular ROS Detection Assay
2.10. Human Subjects
2.11. Measurements in Humans
2.12. Statistical Analyses
3. Results
3.1. Genetic Depletion of Uricase Increases Plasma UA Levels but Does Not Affect Pulmonary Phenotype in Nondiseased WT Mice
3.2. Genetic Depletion of Uox in Elastase-Induced COPD Mice Improves Emphysematous Phenotype in Female-Specific Manner
3.3. Pharmacologically Increased Plasma UA Levels in Chronic COPD Mice Reduce Oxidative Stress and Improve Emphysematous Phenotype and Lung Dysfunction in Female Mice Only
3.4. UA Suppresses Hydrogen Peroxide-Induced Oxidative Stress in Human Lung Epithelial Cells Only in the Presence of Estrogen
3.5. Serum UA Levels Inversely Correlated with Aging-Dependent Decline of Lung Function in a Female-Specific Manner in Humans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes:, P.J. Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2000, 343, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochs-Balcom, H.M.; Grant, B.J.B.; Muti, P.; Sempos, C.T.; Freudenheim, J.L.; Browne, R.W.; Trevisan, M.; Iacoviello, L.; Cassano, P.A.; Schünemann, H.J. Oxidative stress and pulmonary function in the general population. Am. J. Epidemiol. 2005, 162, 1137–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecker, L. Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L642–L653. [Google Scholar] [CrossRef] [PubMed]
- Bentley, A.R.; Kritchevsky, S.B.; Harris, T.B.; Holvoet, P.; Jensen, R.L.; Newman, A.B.; Lee, J.S.; Yende, S.; Bauer, D.; Cassano, P.A. Dietary antioxidants and forced expiratory volume in 1 s decline: The Health, Aging and Body Composition study. Eur. Respir. J. 2012, 39, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.Y.; Lee, C.Y.; Lee, M.G.; Kim, Y.S. Effects of dietary antioxidant vitamins on lung functions according to gender and smoking status in Korea: A population-based cross-sectional study. BMJ Open 2018, 8, e020656. [Google Scholar] [CrossRef]
- van der Vliet, A.; O’Neill, C.; Cross, C.E.; Koostra, J.M.; Volz, W.G.; Halliwell, B.; Louie, S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999, 276, L289–L296. [Google Scholar] [CrossRef] [Green Version]
- Becker, B.F.; Reinholz, N.; Leipert, B.; Raschke, P.; Permanetter, B.; Gerlach, E. Role of uric acid as an endogenous radical scavenger and antioxidant. Chest 1991, 100, 176S–181S. [Google Scholar] [CrossRef] [Green Version]
- Peden, D.B.; Hohman, R.; Brown, M.E.; Mason, R.T.; Berkebile, C.; Fales, H.M.; Kaliner, M.A. Uric acid is a major antioxidant in human nasal airway secretions. Proc. Natl. Acad. Sci. USA 1990, 87, 7638–7642. [Google Scholar] [CrossRef] [Green Version]
- Kratzer, J.T.; Lanaspa, M.A.; Murphy, M.N.; Cicerchi, C.; Graves, C.L.; Tipton, P.A.; Ortlund, E.A.; Johnson, R.J.; Gaucher, E.A. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. USA 2014, 111, 3763–3768. [Google Scholar] [CrossRef] [Green Version]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [Green Version]
- Simic, M.G.; Jovanovic, S.V. Antioxidation mechanisms of uric acid. J Am Chem Soc 1989, 111, 5778–5782. [Google Scholar] [CrossRef]
- Matsuo, H.; Tomiyama, H.; Satake, W.; Chiba, T.; Onoue, H.; Kawamura, Y.; Nakayama, A.; Shimizu, S.; Sakiyama, M.; Funayama, M.; et al. ABCG2 variant has opposing effects on onset ages of Parkinson’s disease and gout. Ann. Clin. Transl. Neurol. 2015, 2, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Scott, G.S.; Zborek, A.; Mikheeva, T.; Kean, R.B.; Koprowski, H.; Spitsin, S.V. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. Fed. Am. Soc. Exp. Biol. 2000, 14, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L.; Shannon, J.; Frei, B.; Kaye, J.A.; Quinn, J.F. Uric acid as a CNS antioxidant. J. Alzheimer’s Dis. 2010, 19, 1331–1336. [Google Scholar] [CrossRef] [Green Version]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef]
- Feig, D.I. The Role of Uric Acid in the Pathogenesis of Hypertension in the Young. J. Clin. Hypertens. 2012, 14, 346–352. [Google Scholar] [CrossRef]
- Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Sung, S.A.; Kim, Y.S.; Oh, K.H.; Ahn, C.; Kim, S.W. Hyperuricemia has increased the risk of progression of chronic kidney disease: propensity score matching analysis from the KNOW-CKD study. Sci. Rep. 2019, 9, 6681. [Google Scholar] [CrossRef]
- Shaheen, S.O. Antioxidants and respiratory disease: The uric acid paradox. Thorax 2014, 69, 978–979. [Google Scholar] [CrossRef] [Green Version]
- Horsfall, L.J.; Nazareth, I.; Petersen, I. Serum uric acid and the risk of respiratory disease: A population-based cohort study. Thorax 2014, 69, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Burdett, T.C.; Desjardins, C.A.; Logan, R.; Cipriani, S.; Xu, Y.; Schwarzschild, M.A. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Kahnert, K.; Alter, P.; Welte, T.; Huber, R.M.; Behr, J.; Biertz, F.; Watz, H.; Bals, R.; Vogelmeier, C.F.; Jörres, R.A. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD: A multi-dimensional approach. Respir. Res. 2018, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate-anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Matsuo, H.; Chiba, T.; Nagamori, S.; Nakayama, A.; Domoto, H.; Phetdee, K.; Wiriyasermkul, P.; Kikuchi, Y.; Oda, T.; Nishiyama, J.; et al. Mutations in Glucose Transporter 9 Gene SLC2A9 Cause Renal Hypouricemia. Am. J. Hum. Genet. 2008, 83, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Nakayama, A.; Ikebuchi, Y.; Ito, K.; Kusanagi, Y.; Chiba, T.; Tadokoro, S.; et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: A function-based genetic analysis in a Japanese population. Sci. Transl. Med. 2009, 1, 5ra11. [Google Scholar] [CrossRef] [PubMed]
- Woodward, O.M.; Köttgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Köttgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuto, T.; Kamei, S.; Nohara, H.; Fujikawa, H.; Tasaki, Y.; Sugahara, T.; Ono, T.; Matsumoto, C.; Sakaguchi, Y.; Kondo, Y.; et al. Pharmacological and genetic reappraisals of protease and oxidative stress pathways in a mouse model of obstructive lung diseases. Sci. Rep. 2016, 6, 39305. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, R.; Kamei, S.; Nohara, H.; Fujikawa, H.; Maruta, K.; Kawakami, T.; Eto, Y.; Takahashi, N.; Suico, M.A.; Takeo, T.; et al. Auto-measure emphysematous parameters and pathophysiological gene expression profiles in experimental mouse models of acute and chronic obstructive pulmonary diseases. J. Pharmacol. Sci. 2019, 140, 113–119. [Google Scholar] [CrossRef]
- Wu, X.; Wakamiya, M.; Vaishnav, S.; Geske, R.; Montgomery, C.; Jones, P.; Bradley, A.; Caskey, C.T. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc. Natl. Acad. Sci. USA 1994, 91, 742–746. [Google Scholar] [CrossRef] [Green Version]
- Takeo, T.; Nakagata, N. Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 2015, 10, e0128330. [Google Scholar] [CrossRef]
- Tanaka, K.I.; Ishihara, T.; Sugizaki, T.; Kobayashi, D.; Yamashita, Y.; Tahara, K.; Yamakawa, N.; Iijima, K.; Mogushi, K.; Tanaka, H.; et al. Mepenzolate bromide displays beneficial effects in a mouse model of chronic obstructive pulmonary disease. Nat. Commun. 2013, 4, 2686. [Google Scholar] [CrossRef] [Green Version]
- Cozens, A.L.; Yezzi, M.J.; Chin, L.; Simon, E.M.; Finkbeiner, W.E.; Wagner, J.A.; Gruenert, D.C. Characterization of immortal cystic fibrosis tracheobronchial gland epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 5171–5175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, S.; Fujikawa, H.; Nohara, H.; Ueno-Shuto, K.; Maruta, K.; Nakashima, R.; Kawakami, T.; Matsumoto, C.; Sakaguchi, Y.; Ono, T.; et al. Zinc deficiency via a splice switch in zinc importer ZIP2/SLC39A2 causes cystic fibrosis-associated MUC5AC hypersecretion in airway epithelial Cells. EBioMedicine 2018, 27, 304–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Yokote, K.; Arai, H.; Iida, M.; Ishigaki, Y.; Ishibashi, S.; Umemoto, S.; Egusa, G.; Ohmura, H.; Okamura, T.; et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J. Atheroscler. Thromb. 2018, 25, 846–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tomioka, N.H.; Watanabe, S.; Suzuki, Y.; Tsuchiya, M.; Hosoyamada, M. The mechanism of false in vitro elevation of uric acid level in mouse blood. Biol. Pharm. Bull. 2016, 39, 1081–1084. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Zhou, J.; Wen, Y.; Li, J.; Cheng, C.H.K. Aesculin possesses potent hypouricemic action in rodents but is devoid of xanthine oxidase/dehydrogenase inhibitory activity. Planta Med. 2002, 68, 175–178. [Google Scholar] [CrossRef]
- Zhang, Y.; Yamamoto, T.; Hisatome, I.; Li, Y.; Cheng, W.; Sun, N.; Cai, B.; Huang, T.; Zhu, Y.; Li, Z.; et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol. Cell. Endocrinol. 2013, 375, 89–96. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol.-Cell Physiol. 2007, 293, C584–C596. [Google Scholar] [CrossRef]
- Verzola, D.; Ratto, E.; Villaggio, B.; Parodi, E.L.; Pontremoli, R.; Garibotto, G.; Viazzi, F. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One 2014, 9, e115210. [Google Scholar] [CrossRef]
- Song, J.U.; Hwang, J.; Ahn, J.K. Serum uric acid is positively associated with pulmonary function in Korean health screening examinees. Mod. Rheumatol. 2017, 27, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Nicks, M.E.; O’Brien, M.M.; Bowler, R.P. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD J. Chronic Obstr. Pulm. Dis. 2011, 8, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Potts, J.F.; Omenaas, E.; Heinrich, J.; Svanes, C.; Garcia-Aymerich, J.; Burney, P.G.; Jarvis, D.L. Dietary antioxidants and 10-year lung function decline in adults from the ECRHS survey. Eur. Respir. J. 2017, 50, 1602286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niikura, Y.; Ishii, T.; Hosoki, K.; Nagase, T.; Yamashita, N. Ovary-dependent emphysema augmentation and osteopontin induction in adult female mice. Biochem. Biophys. Res. Commun. 2015, 461, 642–647. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.P.; Postma, D.S.; Camp, P.; Sin, D.D. Female smokers beyond the perimenopausal period are at increased risk of chronic pulmonary disease: A systematic review and meta-analysis. Respir. Res. 2006, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Carlson, C.L.; Cushman, M.; Enright, P.L.; Cauley, J.A.; Newman, A.B. Hormone replacement therapy is associated with higher FEV1 in elderly women. Am. J. Respir. Crit. Care Med. 2001, 163, 423–428. [Google Scholar] [CrossRef]
- Tatsumi, K.; Kasahara, Y.; Kurosu, K.; Tanabe, N.; Takiguchi, Y.; Kuriyama, T. Clinical phenotypes of COPD: Results of a Japanese epidemiological survey. Respirology 2004, 9, 331–336. [Google Scholar] [CrossRef]
- Do, J.G.; Park, C.-H.; Lee, Y.-T.; Yoon, K.J. Association between underweight and pulmonary function in 282,135 healthy adults: A cross-sectional study in Korean population. Sci. Rep. 2019, 9, 14308. [Google Scholar] [CrossRef]
- Pierik, V.D.; Meskers, C.G.M.; Van Ancum, J.M.; Numans, S.T.; Verlaan, S.; Scheerman, K.; Kruizinga, R.C.; Maier, A.B. High risk of malnutrition is associated with low muscle mass in older hospitalized patients—A prospective cohort study. BMC Geriatr. 2017, 17, 118. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Saltin, B. Exercise as medicine - Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport. 2015, 25, 1–72. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Que, S.; Zhou, L.; Zheng, S. Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: A Meta-analysis of Prospective Studies. Sci. Rep. 2015, 5, 14325. [Google Scholar] [CrossRef] [Green Version]
- Shih, M.H.; Lazo, M.; Liu, S.H.; Bonekamp, S.; Hernaez, R.; Clark, J.M. Association between serum uric acid and nonalcoholic fatty liver disease in the US population. J. Formos. Med. Assoc. 2015, 114, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Xu, C.; Kang, X.; Zhang, S.; Li, H.; Tao, L.; Zheng, D.; Guo, X.; Yang, X. Changing trajectories of serum uric acid and risk of non-alcoholic fatty liver disease: A prospective cohort study. J. Transl. Med. 2020, 18, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Lonardo, A.; Vinco, G.; Zoppini, G.; Lippi, G.; Bonora, E.; Loomba, R.; Tilg, H.; Byrne, C.D.; Fabbri, L.; et al. Association between non-alcoholic fatty liver disease and decreased lung function in adults: A systematic review and meta-analysis. Diabetes Metab. 2019, 45, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.L.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Braga, F.; Pasqualetti, S.; Ferraro, S.; Panteghini, M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2016, 54, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Ota, T.; Tamura, Y.; Chang, W.X.; Shibata, S.; Uchida, S. Time to target uric acid to retard CKD progression. Clin. Exp. Nephrol. 2017, 21, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Uric acid and cardiovascular disease. Clin. Chim. Acta 2018, 484, 150–163. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Kelley, E.E.; Nakagawa, T.; Madero, M.; Feig, D.I.; Borghi, C.; Piani, F.; Cara-Fuentes, G.; Bjornstad, P.; et al. Uric Acid and Hypertension: An Update With Recommendations. Am. J. Hypertens. 2020, in press. [Google Scholar] [CrossRef]
- Wang, G.; Qian, P.; Jackson, F.R.; Qian, G.; Wu, G. Sequential activation of JAKs, STATs and xanthine dehydrogenase/oxidase by hypoxia in lung microvascular endothelial cells. Int. J. Biochem. Cell Biol. 2008, 40, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Bartziokas, K.; Papaioannou, A.I.; Loukides, S.; Papadopoulos, A.; Haniotou, A.; Papiris, S.; Kostikas, K. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur. Respir. J. 2014, 43, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, H.; Nishimura, M.; Shibuya, E.; Makita, H.; Tsujino, I.; Miyamoto, K.; Kawakami, Y. Tissue hypoxia in sleep apnea syndrome assessed by uric acid and adenosine. Chest 2002, 122, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, M.A.; Wynne, K.M.; Badesch, D.B.; Groves, B.M.; Voelkel, N.F. Hyperuricemia in severe pulmonary hypertension. Chest 2000, 117, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, T.; Hamada, H.; Yokoyama, A.; Abe, M.; Nishimura, K.; Kohno, N.; Inata, J.; Kuraoka, T.; Moritani, C.; Higaki, J. Significance of serum uric acid in patients with chronic respiratory failure treated with non-invasive positive pressure ventilation. Intern. Med. 2007, 46, 691–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.S.; Tripathi, V. Smoking under hypoxic conditions: A potent environmental risk factor for inflammatory and autoimmune diseases. Mil. Med. Res. 2018, 5, 11. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Fukuhara, A.; Saito, J.; Sato, S.; Saito, K.; Fukuhara, N.; Tanino, Y.; Wang, X.; Rinno, K.; Suzuki, H.; Munakata, M. The association between risk of airflow limitation and serum uric acid measured at medical health check-ups. Int. J. COPD 2017, 12, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Amaral, A.F.S.; Strachan, D.P.; Burney, P.G.J.; Jarvis, D.L. Female smokers are at greater risk of airflow obstruction than male smokers UK Biobank. Am. J. Respir. Crit. Care Med. 2017, 195, 1226–1235. [Google Scholar] [CrossRef]





| Characteristics | Males | Females | p Value |
|---|---|---|---|
| (n = 356) | (n = 197) | ||
| Age (years) | 66.1 ± 9.0 | 66.8 ± 8.1 | 0.39 |
| BMI (kg/m2) | 23.4 ± 2.7 | 22.4 ± 3.2 | <0.01 |
| FEV1 / FVC (%) | 75.7 ± 6.6 | 77.5 ± 6.2 | <0.01 |
| FEV1 (% predicted) | 93.7 ± 14.0 | 104.1 ± 14.9 | <0.01 |
| FVC (% predicted) | 105.2 ± 15.5 | 108.6 ± 14.7 | 0.01 |
| Smoking status | |||
| Past smoking (%) | 44.4 | 2.5 | <0.01 |
| Current smoking (%) | 15.2 | 0.5 | |
| Smoking exposure (pack-years) | 18.7 ± 22.8 | 0.3 ± 2.1 | <0.01 |
| Drinkers (%) | 70.4 | 27.8 | <0.01 |
| Systolic BP (mmHg) | 122.8 ± 15.7 | 120.4 ± 16.6 | 0.09 |
| Diastolic BP (mmHg) | 73.6 ± 11.2 | 70.1 ± 10.2 | <0.01 |
| LDL-C (mg/dL) | 118.8 ± 26.9 | 124.4 ± 26.2 | 0.02 |
| HDL-C (mg/dL) | 63.4 ± 15.0 | 74.5 ± 17.8 | <0.01 |
| Triglyceride (mg/dL) | 110.7 ± 63.3 | 95.9 ± 44.9 | <0.01 |
| Fasting plasma glucose (mg/dL) | 103.7 ± 18.9 | 96.3 ± 14.3 | <0.01 |
| eGFR (mL/min/1.73m2) | 69.4 ± 12.9 | 74.3 ± 12.7 | <0.01 |
| Uric acid (mg/dL) | 5.8 ± 1.3 | 4.6 ± 1.0 | <0.01 |
| Hypertension (%) | 46.3 | 36.5 | 0.03 |
| Diabetes (%) | 20.2 | 8.6 | <0.01 |
| Dyslipidemia (%) | 52.8 | 58.4 | 0.21 |
| Fatty liver (%) | 24.2 | 14.2 | <0.01 |
| Hyperuricemia (%) | 18.8 | 3.0 | <0.01 |
| SLC2A9 genotype (C/C, C/T, T/T) (%) | 51.1, 41.3, 7.6 | 48.7, 42.1, 9.1 | 0.73 |
| Sex | Uric Acid | Linear Regression Analysis | Bootstrap Evaluation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | B | SE | p value | B | SE | 95% CI | p-Value | ||
| Males | <5 mg/dL | 88 | 0 | - | - | 0 | - | - | - |
| 5–6 mg/dL | 115 | 1.02 | 0.90 | 0.26 | 1.02 | 0.89 | −0.66, 2.82 | 0.26 | |
| 6–7 mg/dL | 84 | 1.01 | 0.97 | 0.30 | 1.01 | 1.08 | −1.07, 3.13 | 0.34 | |
| ≥7 mg/dL | 69 | 0.28 | 1.07 | 0.79 | 0.28 | 1.07 | −1.77, 2.39 | 0.79 | |
| Females | <4 mg/dL | 58 | 0 | - | - | 0 | - | - | - |
| 4–5 mg/dL | 75 | 0.55 | 1.02 | 0.59 | 0.55 | 1.01 | −1.47, 2.74 | 0.55 | |
| 5–6 mg/dL | 48 | 0.16 | 1.19 | 0.89 | 0.16 | 1.19 | −2.23, 2.63 | 0.89 | |
| ≥6 mg/dL | 16 | 3.59 | 1.71 | 0.04 | 3.59 | 1.49 | 0.43, 6.51 | 0.02 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujikawa, H.; Sakamoto, Y.; Masuda, N.; Oniki, K.; Kamei, S.; Nohara, H.; Nakashima, R.; Maruta, K.; Kawakami, T.; Eto, Y.; et al. Higher Blood Uric Acid in Female Humans and Mice as a Protective Factor against Pathophysiological Decline of Lung Function. Antioxidants 2020, 9, 387. https://doi.org/10.3390/antiox9050387
Fujikawa H, Sakamoto Y, Masuda N, Oniki K, Kamei S, Nohara H, Nakashima R, Maruta K, Kawakami T, Eto Y, et al. Higher Blood Uric Acid in Female Humans and Mice as a Protective Factor against Pathophysiological Decline of Lung Function. Antioxidants. 2020; 9(5):387. https://doi.org/10.3390/antiox9050387
Chicago/Turabian StyleFujikawa, Haruka, Yuki Sakamoto, Natsuki Masuda, Kentaro Oniki, Shunsuke Kamei, Hirofumi Nohara, Ryunosuke Nakashima, Kasumi Maruta, Taisei Kawakami, Yuka Eto, and et al. 2020. "Higher Blood Uric Acid in Female Humans and Mice as a Protective Factor against Pathophysiological Decline of Lung Function" Antioxidants 9, no. 5: 387. https://doi.org/10.3390/antiox9050387
APA StyleFujikawa, H., Sakamoto, Y., Masuda, N., Oniki, K., Kamei, S., Nohara, H., Nakashima, R., Maruta, K., Kawakami, T., Eto, Y., Takahashi, N., Takeo, T., Nakagata, N., Watanabe, H., Otake, K., Ogata, Y., Tomioka, N. H., Hosoyamada, M., Takada, T., ... Shuto, T. (2020). Higher Blood Uric Acid in Female Humans and Mice as a Protective Factor against Pathophysiological Decline of Lung Function. Antioxidants, 9(5), 387. https://doi.org/10.3390/antiox9050387