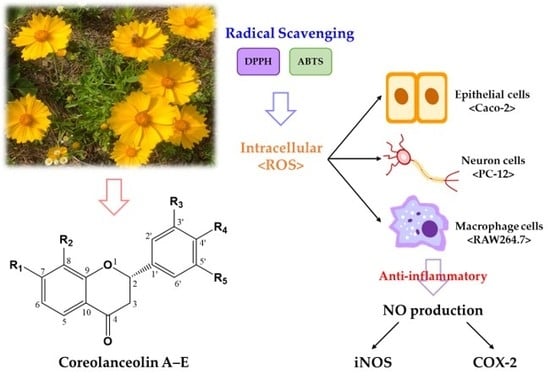
Coreolanceolins A–E, New Flavanones from the Flowers of Coreopsis lanceolate, and Their Antioxidant and Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. General Experimental Procedures
2.3. Extraction and Isolation
2.4. Quantitative Analysis of Compound 1–7 Using Reversed-Phase HPLC
2.5. Antioxidant Activities
2.5.1. Free Radical Scavenging Activity
2.5.2. Cell Culture and Cytotoxicity
2.5.3. Measurement of Intracellular OS
2.6. Pro-Inflammatory Inhibition Activity Assay
2.6.1. Determination of NO Production
2.6.2. Western Blot Analysis for Protein Expression
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Structure Elucidation of Compound 1–7 from CLF
3.2. Quantitative Analysis of Compound 1–7 in the Extracts from CLF
3.3. Radical Scavenging Activities of Compound 1–7 Using DPPH and ABTS Assays
3.4. Inhibition Effects of Compound 1–7 on Intracellular OS in PC-12, Caco-2, and RAW 264.7 Cells
3.5. Inhibitory Effects of Flavanones 1–7 on NO Production in RAW 264.7 Cells
3.6. Inhibitory Effects of Flavanone 1–7 on Expression of iNOS and COX-2 in RAW 264.7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-azino-bis(2-amidinopropane) dihydrochloride |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| AMT | amino-5,6-dihydro-6-methyl-4H-1,3-thiazine hydrochloride |
| AP | amorphous powder |
| CC | column chromatography |
| CD | circular dichroism |
| CE | cotton effect |
| CLF | Coreopsis lanceolata flowers |
| CLFE | Coreopsis lanceolata flowers ethyl acetate fraction |
| CLFB | Coreopsis lanceolata flowers n-butanol fraction |
| CLFW | Coreopsis lanceolata flowers water fraction |
| CMR | carbon-13 nuclear magnetic resonance (13C-NMR) |
| CMRM100 | 13C-NMR (100 MHz, CD3OD, δC) |
| COX-2 | cyclooxygenase-2 |
| DCFH-DA | 2′,7′-dichlorofluorescein diacetate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DW | dry weight |
| EtOAc | ethyl acetate |
| EV/TV | elution volume/total volume |
| FM | Fast atom bombardment mass spectroscopy (FAB-MS) |
| FBS | fetal bovine serum |
| frs. | fractions |
| gHMBC | gradient heteronuclear multiple bond coherence |
| H2O2 | hydrogen peroxide |
| HPLC | high-performance liquid chromatography |
| HR | high resolution |
| HSD | honestly significant difference |
| iNOS | inducible nitric oxide synthase |
| IRS | infrared spectrum |
| MeOH | methanol |
| MF | molecular formula |
| MIP | molecular ion peak |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide |
| NBT | nitro blue tetrazolium |
| n-BuOH | n-butanol |
| NMR | nuclear magnetic resonance |
| NO | nitric oxide |
| OV | overlapped |
| PMR | proton-1 nuclear magnetic resonance (1H-NMR) |
| PMRM400 | 1H-NMR (400 MHz, CD3OD, δH) |
| ROS | reactive oxygen species |
| RPMI | 1640 Roswell Park Memorial Institute 1640 |
| SiO2 | silica gel |
| SO | superoxide |
| ODS | octadecyl silica gel |
| OS | oxidative stress |
| PBS | phosphate-buffered saline |
| TLC | thin layer chromatography |
| UV | ultraviolet |
| UVAC | UV absorption characteristics |
| 1D-NMR | one-dimensional (1D) NMR |
| 2D-NMR | Two-dimensional (2D) NMR |
References
- Panero, J.L.; Funk, V.A. Toward a phylogenetic subfamilial classification for the Compositae (Asteraceae). Proc. Biol. Soc. Wash 2002, 115, 760–773. [Google Scholar]
- Gibbs, R.G.E. List of species of southern African plants. In Memoirs of the Botanical Survey of South Africa, 2nd ed.; Botanical Research Institute, Dept. of Agriculture: Fort Worth, South Africa, 1987; pp. 1–152(1), 1–270(2). [Google Scholar]
- Kim, H.G.; Oh, H.J.; Ko, J.H.; Song, H.S.; Lee, Y.G.; Kang, S.C.; Lee, D.Y.; Baek, N.I. Lanceoleins A–G, hydroxychalcones, from the flowers of Coreopsis lanceolata and their chemopreventive effects against human colon cancer cells. Bioorg. Chem. 2019, 85, 274–281. [Google Scholar] [CrossRef]
- Oyvind, M.A.; Kenneth, R.M. Flavanones and Dihydroflavonols. In Flavonoids: Chemistry, Biochemistry and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 918–919. [Google Scholar]
- Tanimoto, S.; Miyazawa, M.; Inoue, T.; Okada, Y.; Nomura, M. Chemical constituents of Coreopsis lanceolata L. and their physiological activities. J. Oleo. Sci. 2009, 58, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, D.; Zheng, D.; Hu, R.; Chen, W.; Chen, D.; Zhuo, X. Chemical constituents from Coreopsis lanceolata. Zhongcaoyao 2013, 44, 1558–1561. [Google Scholar]
- Pardede, A.; Mashita, K.; Ninomiya, M.; Tanaka, K.; Koketsu, M. Flavonoid profile and antileukemic activity of Coreopsis lanceolata flowers. Bioorg. Med. Chem. Lett. 2016, 26, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Hiraoka, K.; Kawano, T.; Fujioka, S.; Shimada, A. Nematicidal activities of acetylene compounds from Coreopsis lanceolata L. J. Biosci. 2008, 63, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Higashi, N.; Taki, H.; Osawa, T. Cytolytic mechanisms of activated macrophages. Tumor necrosis factor and L-arginine-dependent mechanisms act synergistically as the major cytolytic mechanisms of activated macrophages. J. Immunol. 1990, 144, 1425–1431. [Google Scholar]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Utaipan, T.; Boonyanuphong, P.; Chuprajob, T.; Suksamrarn, A.; Chunglok, W. A trienone analog of curcumin, 1,7-bis(3-hydroxyphenyl)-1,4,6-heptatrien-3-one, possesses ROS- and caspase-mediated apoptosis in human oral squamous cell carcinoma cells in vitro. Appl. Biol. Chem. 2020, 63, 7. [Google Scholar] [CrossRef]
- Kim, J.; Soh, S.Y.; Bae, H.; Nam, S.Y. Antioxidant and phenolic contents in potatoes (Solanum tuberosum L.) and micropropagated potatoes. Appl. Biol. Chem. 2019, 62, 17. [Google Scholar] [CrossRef]
- Rha, C.S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.O. Antioxidative, anti-Inflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, Y.; Okita, M.; Murai, Y.; Okano, Y.; Nomura, M. Isolation and identification of flavonoids from Coreopsis lanceolata L. petals. Nat. Prod. Res. 2014, 8, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, W.; Yang, H.; Xin, Z. Isolation and identification of active ingredients on angiotensin-converting enzyme inhibition from Coreopsis tinctoria Nutt. Nanjing Nongye Daxue Xuebao 2015, 38, 146–151. [Google Scholar]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lim, J.S.; Hahn, D.; Gu, M.J.; Oh, J.; Lee, J.S.; Kim, J.S. Anti-inflammatory and antioxidant effects of 2, 7-dihydroxy-4, 6-dimethoxy phenanthrene isolated from Dioscorea batatas Decne. Appl. Biol. Chem. 2019, 62, 29. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, S.; Yan, L.; Tai, J.; Xiao, Q.; Zou, K.; Zhou, Y.; Wu, J. Separation of flavanone enantiomers and flavanone glucoside diastereomers from Balanophora involucrata Hook. f. by capillary electrophoresis and reversed-phase high-performance liquid chromatography on a C18 column. J. Chromatogr. A 2008, 1185, 117–129. [Google Scholar] [CrossRef]
- Hopia, A.; Heinonen, M. Antioxidant activity of flavonol aglycones and their glycosides in methyl linoleate. J. Am. Oil Chem. Soc. 1999, 76, 139–144. [Google Scholar] [CrossRef]
- Pier-Giorgio, P. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–354. [Google Scholar] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Radical chemistry of flavonoid antioxidants. In Antioxidants in Therapy and Preventive Medicine, 1st ed.; Emerit, I., Ed.; Plenum Press: New York, NY, USA, 1990; pp. 165–170. [Google Scholar]
- Aniya, Y.; Naito, A. Oxidative stress-induced activation of microsomal glutathione S-transferase in isolated rat liver. Biochem. Pharmacol. 1993, 45, 37–42. [Google Scholar] [PubMed]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Cao, W.; Xia, M.; Pan, S.; Xu, X. Study of structure and permeability relationship of flavonoids in Caco-2 cells. Nutrients 2017, 9, 1301. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Liang, F.; Liu, K.; Qaiser, S.; Pan, S.; Xu, X. Structure characteristics for intestinal uptake of flavonoids in Caco-2 cells. Food Res. Int. 2018, 105, 353–360. [Google Scholar] [CrossRef]
- Rice-Evans, A.C.; Miller, J.N.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Marletta, M.A. Mammalian nitrate biosynthesis: Mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. USA 1985, 82, 7738–7742. [Google Scholar] [CrossRef] [Green Version]
- Grisham, M.B.; Granger, D.N.; Lefer, D.J. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: Relevance to ischemic heart disease. Free Radic. Biol. Med. 1998, 25, 404–433. [Google Scholar] [CrossRef]
- Loscalzo, J.; Welch, G. Nitric oxide and its role in the cardiovascular system. Prog. Cardiovasc. Dis. 1995, 38, 87–104. [Google Scholar] [CrossRef]
- Palombella, V.J.; Conner, E.M.; Fuseler, J.W.; Destree, A.; Davis, J.M.; Laroux, F.S.; Wolf, R.E.; Huang, J.; Brand, S.; Elliott, P.J.; et al. Role of the proteasome and NF-kappaB in Streptococcal cell wall-induced polyarthritis. Proc. Natl. Acad. Sci. USA 1998, 95, 15671–15676. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Park, H.J.; Jung, H.A.; Choi, J.S. Estragole exhibits anti-inflammatory activity with the regulation of NF-κB and Nrf-2 signaling pathways in LPS-induced RAW 264.7 cells. Nat. Prod. Sci. 2018, 24, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.T.; Ryu, G.M.; Kwon, B.M.; Lee, W.H.; Suk, K. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: Inhibition of microglial neurotoxicity. Eur. J. Pharmacol. 2008, 588, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Jang, D.; Jung, Y.S.; Oh, H.J.; Oh, S.M.; Lee, Y.G.; Kang, S.C.; Kim, D.O.; Lee, D.Y.; Baek, N.I. Anti-inflammatory effect of flavonoids from Brugmansia arborea L. flowers. J. Microbiol. Biotechnol. 2020, 30, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Nathan, C. The high-output nitric oxide pathway: Role and regulation. J. Leukoc. Biol. 1994, 56, 576–582. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Salinas, G.; Rangasetty, C.U.; Uretsky, F.B.; Birnbaum, Y. The cyclooxygenase 2 (COX-2) story: It’s time to explain, not inflame. J. Cardiovasc. Pharmacol. Ther. 2007, 12, 98–111. [Google Scholar] [CrossRef]







| No. | δH, Coupling Pattern, J in Hz | ||||
|---|---|---|---|---|---|
| 2 1 | 4 2 | 5 1 | 6 1 | 7 1 | |
| 2 | 5.37, dd, 12.6, 2.8 | 5.56, dd, 12.0, 2.4 | 5.43, br. d, 12.0 | 5.43, dd, 12.8, 2.8 | 5.45, dd, 12.4, 2.4 |
| 3 | 2.98, dd, 17.0, 12.6 2.71, dd, 17.0, 2.8 | 3.23, dd, 16.8, 12.0 3.01, dd, 16.8, 2.4 | 3.14, dd, 17.6, 12.0 2.77, br. d, 17.6 | 3.05, dd, 17.0, 12.8 2.79, dd, 17.0, 2.8 | 3.07, dd, 16.8, 12.4 2.81, dd, 16.8, 2.4 |
| 5 | 7.47, d, 8.8 | 7.84, d, 9.2 | 7.34, d, 8.8 | 7.84, d, 9.2 | 7.60, d, 8.8 |
| 6 | 6.48, d, 8.8 | 7.24, d, 9.2 | 6.89, d, 8.8 | 6.81, d, 9.2 | 6.92, d, 8.8 |
| 2′ | 7.00, br. s | 7.53, d, 1.6 | 7.13, br. s | 7.23, br. s | 7.00, br. s |
| 4′ | 6.94, br. s | - | - | - | 6.94, br. s |
| 5′ | - | 7.59, d, 8.0 | 6.79, d, 8.0 | 6.89, d, 8.0 | - |
| 6′ | 6.94, br. s | 7.09, dd, 8.0, 1.6 | 6.93, br. d, 8.0 | 6.93, br. d, 8.0 | 6.94, br. s |
| 8-OCH3 | 3.79, s | 3.87, s | - | 3.83, s | 3.84, s |
| 3′-OCH3 | - | - | 3.85, s | - | - |
| 4′-OCH3 | - | - | - | 3.86, s | - |
| 5′-OCH3 | 3.86, s | - | - | - | 3.86, s |
| 1″ | - | 5.68, d, 7.0 | 4.93, d, 7.2 | 5.06, d, 8.0 | 5.04, d, 7.2 |
| 2″ | - | 4.34 #3 | 3.52 # | 3.53 # | 3.53 # |
| 3″ | - | 4.13 # | 3.38 # | 3.36 # | 3.36 # |
| 4″ | - | 4.25 # | 3.41 # | 3.42 # | 3.41 # |
| 5″ | - | 4.30 # | 3.50 # | 3.47 # | 3.47 # |
| 6″ | - | 4.50, br. d, 12.0 4.14 # | 3.89, br. d, 12.0 3.81, dd, 12.0, 5.6 | 3.89, br. d, 12.0 3.70, dd, 12.0, 5.6 | 3.89, br. d, 12.8 3.69, dd, 12.8, 5.6 |
| No. | δC | ||||
|---|---|---|---|---|---|
| 2 1 | 4 2 | 5 1 | 6 1 | 7 1 | |
| 2 | 81.0 | 80.8 | 81.6 | 81.4 | 81.2 |
| 3 | 44.8 | 44.4 | 44.9 | 45.0 | 44.9 |
| 4 | 193.1 | 191.3 | 194.2 | 194.2 | 193.4 |
| 5 | 123.9 | 122.7 | 118.7 | 122.3 | 123.4 |
| 6 | 113.4 | 110.0 | 110.7 | 107.6 | 110.9 |
| 7 | 159.8 | 157.3 | 152.9 | 157.3 | 158.0 |
| 8 | 137.2 | 138.4 | 133.4 | 138.1 | 138.0 |
| 9 | 161.0 | 156.3 | 152.4 | 159.0 | 160.6 |
| 10 | 114.2 | 117.6 | 115.9 | 117.6 | 118.2 |
| 1′ | 133.6 | 130.9 | 130.8 | 132.7 | 133.1 |
| 2′ | 113.4 | 115.0 | 111.6 | 114.6 | 114.6 |
| 3′ | 147.8 | 147.1 | 148.1 | 147.8 | 147.8 |
| 4′ | 112.7 | 147.5 | 149.1 | 149.3 | 112.6 |
| 5′ | 149.3 | 116.4 | 116.0 | 115.2 | 149.4 |
| 6′ | 118.8 | 118.6 | 120.6 | 119.1 | 118.2 |
| 1″ | - | 101.8 | 102.9 | 101.7 | 101.9 |
| 2″ | - | 74.5 | 74.7 | 74.7 | 74.8 |
| 3″ | - | 78.7 | 78.4 | 78.3 | 78.3 |
| 4″ | - | 70.9 | 71.2 | 70.9 | 71.2 |
| 5″ | - | 77.9 | 77.5 | 77.6 | 78.0 |
| 6″ | - | 62.1 | 62.7 | 62.7 | 62.4 |
| 8-OCH3 | 61.1 | 60.9 | - | 61.3 | 61.7 |
| 3′-OCH3 | - | - | 56.4 | - | - |
| 4′-OCH3 | - | - | - | 56.2 | - |
| 5′-OCH3 | 56.4 | - | - | - | 56.7 |
| Compound | Retention Time (min) | Regression Equation | r2 | Concentration |
|---|---|---|---|---|
| 1 | 17.99 | y = 5008x + 25457 | 0.999 | 5.4 ± 0.3 |
| 2 | 24.20 | y = 6074.7x + 17458 | 0.999 | 3.9 ± 1.1 |
| 3 | 8.78 | y = 20438x + 40392 | 0.999 | 11.3 ± 0.1 |
| 4 | 12.99 | y = 19111x + 63019 | 0.998 | 38.8 ± 0.3 |
| 5 | 13.58 | y = 11101x + 2790.1 | 0.999 | 5.2 ± 0.2 |
| 6 | 18.67 | y = 19952x + 13540 | 0.999 | 2.5 ± 0.2 |
| 7 | 18.67 | y = 12915x − 10826 | 0.999 | 0.8 ± 0.1 |
| Compound | DPPH Radical (mg VCE 1/1000 mg) | ABTS Radical (mg VCE/1000 mg) 1 |
|---|---|---|
| 1 | 104.3 ± 1.9 a2 | 1278.6 ± 26.8 a |
| 2 | 85.0 ± 4.0 b | 1095.7 ± 0.1 b |
| 3 | 76.5 ± 3.2 c | 624.8 ± 3.1 c |
| 4 | 68.8 ± 0.8 d | 610.8 ± 3.9 c |
| 5 | 29.0 ± 0.1 e | 519.2 ± 0.7 d |
| 6 | 27.1 ± 1.3 e | 456.5 ± 0.5 e |
| 7 | 20.5 ± 0.3 f | 325.6 ± 0.2 f |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-G.; Jung, Y.S.; Oh, S.M.; Oh, H.-J.; Ko, J.-H.; Kim, D.-O.; Kang, S.C.; Lee, Y.-G.; Lee, D.Y.; Baek, N.-I. Coreolanceolins A–E, New Flavanones from the Flowers of Coreopsis lanceolate, and Their Antioxidant and Anti-Inflammatory Effects. Antioxidants 2020, 9, 539. https://doi.org/10.3390/antiox9060539
Kim H-G, Jung YS, Oh SM, Oh H-J, Ko J-H, Kim D-O, Kang SC, Lee Y-G, Lee DY, Baek N-I. Coreolanceolins A–E, New Flavanones from the Flowers of Coreopsis lanceolate, and Their Antioxidant and Anti-Inflammatory Effects. Antioxidants. 2020; 9(6):539. https://doi.org/10.3390/antiox9060539
Chicago/Turabian StyleKim, Hyoung-Geun, Young Sung Jung, Seon Min Oh, Hyun-Ji Oh, Jung-Hwan Ko, Dae-Ok Kim, Se Chan Kang, Yeong-Geun Lee, Dae Young Lee, and Nam-In Baek. 2020. "Coreolanceolins A–E, New Flavanones from the Flowers of Coreopsis lanceolate, and Their Antioxidant and Anti-Inflammatory Effects" Antioxidants 9, no. 6: 539. https://doi.org/10.3390/antiox9060539
APA StyleKim, H.-G., Jung, Y. S., Oh, S. M., Oh, H.-J., Ko, J.-H., Kim, D.-O., Kang, S. C., Lee, Y.-G., Lee, D. Y., & Baek, N.-I. (2020). Coreolanceolins A–E, New Flavanones from the Flowers of Coreopsis lanceolate, and Their Antioxidant and Anti-Inflammatory Effects. Antioxidants, 9(6), 539. https://doi.org/10.3390/antiox9060539