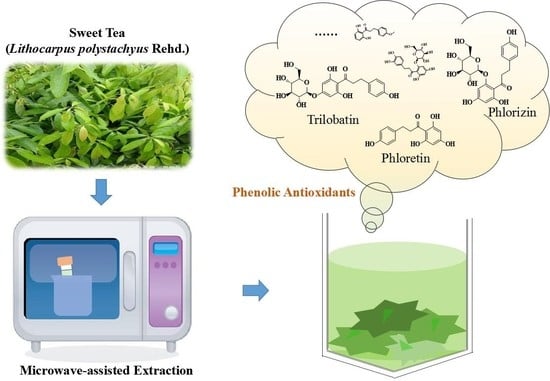
Effects of Microwave-Assisted Extraction Conditions on Antioxidant Capacity of Sweet Tea (Lithocarpus polystachyus Rehd.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sample and Reagents
2.2. Microwave-Assisted Extraction (MAE)
2.3. Maceration Extraction (ME)
2.4. Antioxidant Activity Assays
2.4.1. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Determination of Total Phenolic Content (TPC)
2.6. Experimental Design and Statistical Analysis
2.6.1. Single-Factor Tests
2.6.2. Response Surface Methodology (RSM)
2.7. High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Single-Factor Tests
3.1.1. Effects of Ethanol Concentration
3.1.2. Effects of Solvent-to-Sample Ratio
3.1.3. Effects of Extraction Temperature
3.1.4. Effects of Microwave Power
3.1.5. Effects of Extraction Time
3.2. Analysis of Response Surface Methodology (RSM) Experiments
3.2.1. Results of Central Composite Design (CCD)
3.2.2. Fitting the Models and Statistical Analysis
3.2.3. Effects of Independent Variables on Antioxidant Activity
3.2.4. Effects of Independent Variables on TPC
3.2.5. Verification of the Model
3.3. Identification of Phenolic Compounds in L. Polystachyus Leaf Extract
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Z.H.; Zhang, R.J.; Wu, J.; Zhao, W.M. New dihydrochalcone glycosides from Lithocarpus litseifolius and the phenomenon of C-H -> C-D exchange observed in NMR spectra of phenolic components. J. Asian Nat. Prod. Res. 2009, 11, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Zeng, X.; Huang, S.; Hou, S.Z.; Lai, X.P. Characterization of phenolic constituents in Lithocarpus polystachyus. Anal. Methods 2014, 6, 1359–1363. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.Y.; Yang, Z.; Zhou, C.; Hu, X.J. Identification and quantitative evaluation of major sweet ingredients in sweet tea (Lithocarpus polystachyus Rehd.) based upon location, harvesting time, leaf age. J. Chem. Soc. Pak. 2018, 40, 158–164. [Google Scholar]
- Gao, J.M.; Xu, Y.S.; Zhang, J.Y.; Shi, J.S.; Gong, Q.H. Lithocarpus polystachyus Rehd. leaves aqueous extract protects against hydrogen peroxide-induced SH-SY5Y cells injury through activation of Sirt3 signaling pathway. Int. J. Mol. Med. 2018, 42, 3485–3494. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.T.; Huang, W.C.; Liou, C.J. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef]
- Park, A.; Jeong, H.H.; Lee, J.; Lee, C.S. The inhibitory effect of phloretin on the formation of Escherichia coli O157:H7 biofilm in a microfluidic system. Biochip J. 2012, 6, 299–305. [Google Scholar] [CrossRef]
- Meng, Y.; Ding, L.; Wang, Y.; Nie, Q.T.; Xing, Y.Y.; Ren, Q. Phytochemical identification of Lithocarpus polystachyus extracts by ultra-high-performance liquid chromatography-quadrupole time-of-flight-MS and their protein tyrosine phosphatase 1B and alpha-glucosidase activities. Biomed. Chromatogr. 2020, 34, e4705. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, L.; Wang, H.; Fang, S.T.; Zhang, Q.B.; Yang, L.Q.; Guo, H.J.; Lin, P.; Zhang, J.; Wang, X.J. Lithocarpus Polystachyus Rehd leaf aqueous extract inhibits human breast cancer growth in vitro and in vivo. Nutr. Cancer 2014, 66, 613–624. [Google Scholar] [CrossRef]
- Hou, S.Z.; Xu, S.J.; Jiang, D.X.; Chen, S.X.; Wang, L.L.; Huang, S.; Lai, X.P. Effect of the flavonoid fraction of Lithocarpus polystachyus Rehd. on spontaneously hypertensive and normotensive rats. J. Ethnopharmacol. 2012, 143, 441–447. [Google Scholar] [CrossRef]
- Ravanfar, R.; Moein, M.; Niakousari, M.; Tamaddon, A. Extraction and fractionation of anthocyanins from red cabbage: ultrasonic-assisted extraction and conventional percolation method. J. Food Meas. Charact. 2018, 12, 2271–2277. [Google Scholar] [CrossRef]
- Cujic, N.; Savikin, K.; Jankovic, T.; Pljevljakusic, D.; Zdunic, G.; Ibric, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Zhang, J.J.; Li, Y.; Meng, X.; Li, H.B. Microwave-assisted extraction of phenolic compounds from Melastoma sanguineum fruit: optimization and identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, A.A.; Dordevic, V.B.; Zdunic, G.M.; Pljevljakusic, D.S.; Savikin, K.P.; Godevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Martino, E.; Ramaiola, E.; Urbano, M.; Bracco, F.; Collina, S. Microwave-assisted extraction of coumarin and related compounds from Melilotus officinalis (L.) Pallas as an alternative to Soxhlet and ultrasound-assisted extraction. J. Chromatogr. A 2006, 1125, 147–151. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Meng, J.M.; Mao, Q.Q.; Shang, A.; Li, B.Y.; Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; et al. Effects of tannase and ultrasound treatment on the bioactive compounds and antioxidant activity of green tea extract. Antioxidants 2019, 8, 362. [Google Scholar] [CrossRef] [Green Version]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trac-Trend. Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, G.; Zhao, G.; Hu, X. Optimization of microwave-assisted extraction of astaxanthin from Haematococcus Pluvialis by response surface methodology and antioxidant activities of the extracts. Sep. Sci. Technol. 2009, 44, 243–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Li, Y.; Chi, R. Optimization of ionic liquid-based microwave-assisted extraction of isoflavones from Radix puerariae by response surface methodology. Sep. Purif. Technol. 2014, 129, 71–79. [Google Scholar] [CrossRef]
- Ameer, K.; Bae, S.W.; Jo, Y.; Lee, H.G.; Ameer, A.; Kwon, J.H. Optimization of microwave-assisted extraction of total extract, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves, using response surface methodology (RSM) and artificial neural network (ANN) modelling. Food Chem. 2017, 229, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Gliszczynska-Swiglo, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Dong, H.Q.; Li, M.; Zhu, F.; Liu, F.L.; Huang, J.B. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against alpha-glucosidase and alpha-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Sun, Y.S.; Li, W.; Liu, Z.B. Preparative isolation, quantification and antioxidant activity of dihydrochalcones from Sweet Tea (Lithocarpus polystachyus Rehd.). J. Chromatogr. B 2015, 1002, 372–378. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Radojkovic, M.; Moreira, M.M.; Soares, C.; Barroso, M.F.; Cvetanovic, A.; Svarc-Gajic, J.; Morais, S.; Delerue-Matos, C. Microwave-assisted extraction of phenolic compounds from Morus nigra leaves: optimization and characterization of the antioxidant activity and phenolic composition. J. Chem. Technol. Biotechnol. 2018, 93, 1684–1693. [Google Scholar] [CrossRef] [Green Version]
- Florez, N.; Conde, E.; Dominguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Lin, S.J.; Zhang, J.J.; Zhao, C.N.; Li, H.B. Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris Fruit: Optimization and identification of phenolic compounds. Molecules 2017, 22, 1481. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, R.V.; Chirumamila, R.R.; Marchant, R.; Nigam, P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. J. 2001, 9, 17–23. [Google Scholar] [CrossRef]
- Cheon, D.; Kim, J.; Jeon, D.; Shin, H.C.; Kim, Y. Target proteins of phloretin for its anti-inflammatory and antibacterial activities against Propionibacterium acnes-induced skin infection. Molecules 2019, 24, 1319. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.F.; Huang, Y.M.; Li, K.X.; Chen, Y.Y.; Vanegas, D.; McLamore, E.S.; Shen, Y.B. Leaf extract from Lithocarpus polystachyus Rehd. promote glycogen synthesis in T2DM mice. PLoS ONE 2016, 11, e0166557. [Google Scholar] [CrossRef] [Green Version]
- Saraswati, S.; Alhaider, A.; Abdelgadir, A.M.; Tanwer, P.; Korashy, H.M. Phloretin attenuates STAT-3 activity and overcomes sorafenib resistance targeting SHP-1-mediated inhibition of STAT3 and Akt/VEGFR2 pathway in hepatocellular carcinoma. Cell Commun. Signal. 2019, 17, 127. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Wang, L.X.; Dong, S.S.; Hong, Y.; Zhou, X.H.; Zheng, W.W.; Zheng, C. Phlorizin exerts direct protective effects on palmitic acid (PA)-induced endothelial dysfunction by activating the PI3K/AKT/eNOS signaling pathway and increasing the levels of nitric oxide (NO). Med. Sci. Monit. Basic Res. 2018, 24, 1–9. [Google Scholar] [CrossRef]
- Ren, D.Y.; Liu, Y.F.; Zhao, Y.; Yang, X.B. Hepatotoxicity and endothelial dysfunction induced by high choline diet and the protective effects of phloretin in mice. Food Chem. Toxicol. 2016, 94, 203–212. [Google Scholar] [CrossRef]





| Independent Variable | Units | Code levels | ||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||
| Solvent-to-sample ratio (X1) | mL/g | 13.18 | 20 | 30 | 40 | 46.82 |
| Extraction time (X2) | min | 3.18 | 10 | 20 | 30 | 36.82 |
| Ethanol concentration (X3) | %, v/v | 43.18 | 50 | 60 | 70 | 76.82 |
| Time (min) | Solution A | Solution B |
|---|---|---|
| 0 | 35 | 65 |
| 8 | 70 | 30 |
| 15 | 60 | 40 |
| 25 | 35 | 65 |
| Run | X1 | X2 | X3 | Y (Actual Response Values) | ||
|---|---|---|---|---|---|---|
| Solvent-to-Sample Ratio | Extraction Time | Ethanol Concentration | FRAP | TEAC | TPC | |
| mL/g | min | %, v/v | µmoL Fe(II)/g DW | μmoL Trolox/g DW | mg GAE/g DW | |
| 1 | 30 (0) | 3.18 (−1.68) | 60 (0) | 329.33 | 567.71 | 117.85 |
| 2 | 40 (1) | 10 (−1) | 70 (1) | 284.49 | 582.04 | 112.11 |
| 3 | 20 (−1) | 10 (−1) | 70 (1) | 325.84 | 539.27 | 112.94 |
| 4 | 20 (−1) | 30 (1) | 50 (−1) | 340.12 | 479.63 | 108.70 |
| 5 | 30 (0) | 20 (0) | 43.18 (−1.68) | 372.13 | 574.74 | 119.58 |
| 6 | 30 (0) | 20 (0) | 76.82 (1.68) | 285.69 | 591.00 | 120.48 |
| 7 | 30 (0) | 20 (0) | 60 (0) | 378.74 | 593.92 | 131.03 |
| 8 | 20 (−1) | 30 (1) | 70 (1) | 333.03 | 514.36 | 123.67 |
| 9 | 30 (0) | 20 (0) | 60 (0) | 380.39 | 622.61 | 131.77 |
| 10 | 40 (1) | 10 (−1) | 50 (−1) | 359.64 | 549.56 | 125.76 |
| 11 | 30 (0) | 20 (0) | 60 (0) | 379.84 | 596.59 | 132.22 |
| 12 | 40 (1) | 30 (1) | 70 (1) | 336.88 | 593.50 | 127.40 |
| 13 | 30 (0) | 20 (0) | 60 (0) | 373.23 | 598.07 | 133.08 |
| 14 | 30 (0) | 36.82 (1.68) | 60 (0) | 372.89 | 600.03 | 127.33 |
| 15 | 46.82 (1.68) | 20 (0) | 60 (0) | 359.37 | 553.54 | 128.57 |
| 16 | 30 (0) | 20 (0) | 60 (0) | 369.93 | 610.85 | 131.77 |
| 17 | 20 (−1) | 10 (−1) | 50 (−1) | 377.50 | 515.30 | 122.92 |
| 18 | 40 (1) | 30 (1) | 50 (−1) | 369.78 | 605.10 | 125.76 |
| 19 | 30 (0) | 20 (0) | 60 (0) | 390.85 | 598.88 | 135.79 |
| 20 | 13.18 (−1.68) | 20 (0) | 60 (0) | 360.03 | 445.12 | 116.71 |
| Response Value | Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|---|
| FRAP | Model | 16,692.36 | 9 | 1854.71 | 27.52 | <0.0001 | significant |
| Residual | 673.94 | 10 | 67.39 | ||||
| Lack of Fit | 415.41 | 5 | 83.08 | 1.61 | 0.3077 | not significant | |
| Pure Error | 258.53 | 5 | 51.71 | ||||
| Cor Total | 17,366.30 | 19 | |||||
| R2 | 0.9612 | ||||||
| R2Adj | 0.9263 | ||||||
| C.V.% | 2.32 | ||||||
| TEAC | Model | 40,446.63 | 9 | 4494.07 | 31.18 | <0.0001 | |
| Residual | 1441.35 | 10 | 144.13 | ||||
| Lack of Fit | 831.80 | 5 | 166.36 | 1.36 | 0.3707 | not significant | |
| Pure Error | 609.55 | 5 | 121.91 | ||||
| Cor Total | 41,887.98 | 19 | |||||
| R2 | 0.9656 | ||||||
| R2Adj | 0.9346 | ||||||
| C.V.% | 2.12 | ||||||
| TPC | Model | 1086.89 | 9 | 120.77 | 28.46 | <0.0001 | significant |
| Residual | 42.43 | 10 | 4.24 | ||||
| Lack of Fit | 28.02 | 5 | 5.60 | 1.94 | 0.2416 | not significant | |
| Pure Error | 14.41 | 5 | 2.88 | ||||
| Cor Total | 1129.32 | 19 | |||||
| R2 | 0.9624 | ||||||
| R2Adj | 0.9286 | ||||||
| C.V.% | 1.66 |
| Model Parameter | FRAP | TEAC | TPC | |||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| X1 | −1.96 | 0.3978 d | 33.97 | <0.0001 a | 3.13 | 0.0002 a |
| X2 | 7.73 | 0.0059 b | 4.45 | 0.2007 d | 2.03 | 0.0045 b |
| X3 | −22.86 | <0.0001 a | 7.83 | 0.0367 c | −0.40 | 0.4862 d |
| X1X2 | 11.59 | 0.0025 b | 15.95 | 0.0037 b | 2.35 | 0.0091 b |
| X1X3 | −6.16 | 0.0596 d | −4.73 | 0.2914 d | −2.13 | 0.0154 c |
| X2X3 | 10.85 | 0.0039 b | −4.17 | 0.3495 d | 5.03 | <0.0001 a |
| X12 | −7.55 | 0.0058 b | −37.90 | <0.0001 a | −3.78 | <0.0001 a |
| X22 | −10.58 | 0.0006 a | −8.01 | 0.0298 c | −3.79 | <0.0001 a |
| X32 | −18.43 | <0.0001 a | −8.36 | 0.0246 c | −4.70 | <0.0001 a |
| Intercept | 378.95 | 603.65 | 132.65 | |||
| Response Values | Extraction Methods | ||
|---|---|---|---|
| Predicted | Actual | Maceration | |
| FRAP (μM Fe(II)/g DW) | 383.23 | 381.29 ± 4.42 | 281.82 ± 9.21 |
| TEAC (μM Trolox/g DW) | 614.52 | 613.11 ± 9.32 | 540.8 ± 7.51 |
| TPC (mg GAE/g DW) | 133.62 | 135.94 ± 0.52 | 90.59 ± 0.67 |
| Number | Phenolic Compounds | Retention Time (min) | Maximum Absorption (nm) | Contents (mg/g DW) |
|---|---|---|---|---|
| 1 | Phlorizin | 8.33 | 284.5 | 23.87 ± 0.19 |
| 2 | Trilobatin | 9.13 | 282.1 | 164.38 ± 0.15 |
| 3 | Phloretin | 10.78 | 286.9 | 1.44 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, A.; Luo, M.; Gan, R.-Y.; Xu, X.-Y.; Xia, Y.; Guo, H.; Liu, Y.; Li, H.-B. Effects of Microwave-Assisted Extraction Conditions on Antioxidant Capacity of Sweet Tea (Lithocarpus polystachyus Rehd.). Antioxidants 2020, 9, 678. https://doi.org/10.3390/antiox9080678
Shang A, Luo M, Gan R-Y, Xu X-Y, Xia Y, Guo H, Liu Y, Li H-B. Effects of Microwave-Assisted Extraction Conditions on Antioxidant Capacity of Sweet Tea (Lithocarpus polystachyus Rehd.). Antioxidants. 2020; 9(8):678. https://doi.org/10.3390/antiox9080678
Chicago/Turabian StyleShang, Ao, Min Luo, Ren-You Gan, Xiao-Yu Xu, Yu Xia, Huan Guo, Yi Liu, and Hua-Bin Li. 2020. "Effects of Microwave-Assisted Extraction Conditions on Antioxidant Capacity of Sweet Tea (Lithocarpus polystachyus Rehd.)" Antioxidants 9, no. 8: 678. https://doi.org/10.3390/antiox9080678
APA StyleShang, A., Luo, M., Gan, R.-Y., Xu, X.-Y., Xia, Y., Guo, H., Liu, Y., & Li, H.-B. (2020). Effects of Microwave-Assisted Extraction Conditions on Antioxidant Capacity of Sweet Tea (Lithocarpus polystachyus Rehd.). Antioxidants, 9(8), 678. https://doi.org/10.3390/antiox9080678