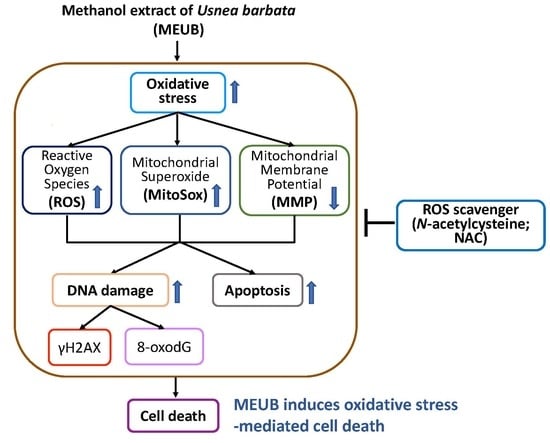
Methanol Extract of Usnea barbata Induces Cell Killing, Apoptosis, and DNA Damage against Oral Cancer Cells through Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Materials
2.2. Extraction and Partition of Methanol Extract of U. barbata (MEUB)
2.3. HPLC Analysis of MEUB
2.4. Determination of the Usnic Acid Content in MEUB
2.5. Cell Culture and Free Radical Scavenger
2.6. Cell Viability
2.7. Cell Cycle Assay
2.8. Annexin V/7AAD Apoptosis Assay
2.9. Pancaspase Apoptosis Assay
2.10. Western Blotting for Apoptosis Signaling
2.11. ROS Assay
2.12. Mitochondrial Superoxide (MitoSOX) Assay
2.13. Mitochondrial Membrane Potential (MMP) Assay
2.14. γH2AX DNA Damage Assay
2.15. 8-oxo-2′deoxyguanosine (8-oxodG) DNA Damage Assay
2.16. Statistics
3. Results
3.1. HPLC Analysis of MEUB
3.2. MEUB Sensitively Kills Oral Cancer Cells rather than Normal Oral Cells
3.3. MEUB Shows SubG1 Changes in Oral Cancer Cells
3.4. MEUB Shows Annexin V/7AAD-Detected Apoptosis in Oral Cancer Cells
3.5. MEUB Shows Pancaspase-Detected Apoptosis in Oral Cancer Cells
3.6. MEUB Activates Apoptosis Signaling in Oral Cancer Cells
3.7. MEUB Shows ROS Induction in Oral Cancer Cells
3.8. MEUB Shows Superoxide Induction in Oral Cancer Cells
3.9. MEUB Shows MMP Reduction in Oral Cancer Cells
3.10. MEUB Shows γH2AX DNA Damage Induction in Oral Cancer Cells
3.11. MEUB Shows 8-OxodG DNA Damage Induction in Oral Cancer Cells
4. Discussion
4.1. Comparison of Drug Sensitivity between Different Usnea Species Extracts to Cancer Cells
4.2. MEUB Exhibits a Preferential Killing Effect to Oral Cancer Cells
4.3. MEUB Triggers Oxidative Stress on Oral Cancer Cells
4.4. MEUB Triggers Apoptosis and DNA Damage on Oral Cancer Cells
4.5. NAC Recovers MEUB-Induced Apoptosis, Oxidative Stress, and DNA Damage on Oral Cancer Cells
4.6. Compounds of Usnea Lichens with Anticancer Effect
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silverman, S., Jr. Oral cancer: Complications of therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 122–126. [Google Scholar] [CrossRef]
- Laraia, L.; Robke, L.; Waldmann, H. Bioactive compound collections: From design to target identification. Chem 2018, 4, 705–730. [Google Scholar] [CrossRef]
- Yen, C.Y.; Hou, M.F.; Yang, Z.W.; Tang, J.Y.; Li, K.T.; Huang, H.W.; Huang, Y.H.; Lee, S.Y.; Fu, T.F.; Hsieh, C.Y.; et al. Concentration effects of grape seed extracts in anti-oral cancer cells involving differential apoptosis, oxidative stress, and DNA damage. BMC Complement. Altern. Med. 2015, 15, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.Y.; Peng, S.Y.; Cheng, Y.B.; Wang, C.L.; Farooqi, A.A.; Yu, T.J.; Hou, M.F.; Wang, S.C.; Yen, C.H.; Chan, L.P.; et al. Ethyl acetate extract of Nepenthes adrianii x clipeata induces antiproliferation, apoptosis, and DNA damage against oral cancer cells through oxidative stress. Environ. Toxicol. 2019, 34, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Wu, J.; Ye, F.; Xue, L.; Jiang, S.; Yi, J.; Zhang, W.; Wei, H.; Sung, M.; Wang, W.; et al. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 2003, 63, 4037–4043. [Google Scholar]
- Kang, H.J.; Jang, Y.J. Selective apoptotic effect of Zelkova serrata twig extract on mouth epidermoid carcinoma through p53 activation. Int. J. Oral Sci. 2012, 4, 78–84. [Google Scholar] [CrossRef]
- Crawford, S.D. Lichens used in traditional medicine. In Lichen Secondary Metabolites; Ranković, B., Ed.; Springer: Berlin, Germany, 2019; pp. 31–97. [Google Scholar]
- Koptina, A.; Shcherbakova, A.; Soldati, F.; Ulrich-Merzenich, G. Total phenolic content and antioxidant capacity of lichen extracts. Z. Phytother. 2013, 34, P35. [Google Scholar] [CrossRef]
- Fernandez-Moriano, C.; Gomez-Serranillos, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Ozturk, S.; Erkisa, M.; Oran, S.; Ulukaya, E.; Celikler, S.; Ari, F. Lichens exerts an anti-proliferative effect on human breast and lung cancer cells through induction of apoptosis. Drug Chem. Toxicol. 2019, 1–9. [Google Scholar] [CrossRef]
- Emsen, B.; Ozdemir, O.; Engin, T.; Togar, B.; Cavusoglu, S.; Turkez, H. Inhibition of growth of U87MG human glioblastoma cells by Usnea longissima Ach. An. Acad. Bras. Cienc. 2019, 91, e20180994. [Google Scholar] [CrossRef] [PubMed]
- Ari, F.; Aztopal, N.; Oran, S.; Bozdemir, S.; Celikler, S.; Ozturk, S.; Ulukaya, E. Parmelia sulcata Taylor and Usnea filipendula Stirt induce apoptosis-like cell death and DNA damage in cancer cells. Cell Prolif. 2014, 47, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zugic, A.; Jeremic, I.; Isakovic, A.; Arsic, I.; Savic, S.; Tadic, V. Evaluation of anticancer and antioxidant activity of a commercially available CO2 supercritical extract of Old Man’s Beard (Usnea barbata). PLoS ONE 2016, 11, e0146342. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; De Feo, V.; Ayala-Zavala, F.J.; Gomes-Cruz, A.; Granato, D.; Coppola, R. Effect of polyphenols on microbial cell-cell communications. In Quorum Sensing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–223. [Google Scholar]
- Wu, S.F.; Hsieh, P.W.; Wu, C.C.; Lee, C.L.; Chen, S.L.; Lu, C.Y.; Wu, T.S.; Chang, F.R.; Wu, Y.C. Camptothecinoids from the seeds of Taiwanese Nothapodytes foetida. Molecules 2008, 13, 1361–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.Y.; Meng, C.L. Regulation of PG synthase by EGF and PDGF in human oral, breast, stomach, and fibrosarcoma cancer cell lines. J. Dent. Res. 1994, 73, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Hung, J.H.; Chen, C.Y.; Omar, H.A.; Huang, K.Y.; Tsao, C.C.; Chiu, C.C.; Chen, Y.L.; Chen, P.H.; Teng, Y.N. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ. Toxicol. 2016, 31, 1888–1898. [Google Scholar] [CrossRef]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef]
- Yeh, C.C.; Tseng, C.N.; Yang, J.I.; Huang, H.W.; Fang, Y.; Tang, J.Y.; Chang, F.R.; Chang, H.W. Antiproliferation and induction of apoptosis in Ca9-22 oral cancer cells by ethanolic extract of Gracilaria tenuistipitata. Molecules 2012, 17, 10916–10927. [Google Scholar] [CrossRef] [Green Version]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W.; Tang, J.Y.; Ou-Yang, F.; Wang, H.R.; Guan, P.Y.; Huang, C.Y.; Chen, C.Y.; Hou, M.F.; Sheu, J.H.; Chang, H.W. Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules 2018, 23, 849. [Google Scholar] [CrossRef] [Green Version]
- Poreba, M.; Strozyk, A.; Salvesen, G.S.; Drag, M. Caspase substrates and inhibitors. Cold Spring Harb. Perspect. Biol. 2013, 5, a008680. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Yen, C.Y.; Wang, H.R.; Yang, H.P.; Tang, J.Y.; Huang, H.W.; Hsu, S.H.; Chang, H.W. Tenuifolide B from Cinnamomum tenuifolium stem selectively inhibits proliferation of oral cancer cells via apoptosis, ROS generation, mitochondrial depolarization, and DNA damage. Toxins 2016, 8, 319. [Google Scholar] [CrossRef]
- Yeh, C.C.; Yang, J.I.; Lee, J.C.; Tseng, C.N.; Chan, Y.C.; Hseu, Y.C.; Tang, J.Y.; Chuang, L.Y.; Huang, H.W.; Chang, F.R.; et al. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement. Altern. Med. 2012, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.T.; Huang, C.Y.; Tang, J.Y.; Liaw, C.C.; Li, R.N.; Liu, J.R.; Sheu, J.H.; Chang, H.W. Reactive oxygen species mediate soft corals-derived sinuleptolide-induced antiproliferation and DNA damage in oral cancer cells. OncoTargets Ther. 2017, 10, 3289–3297. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.S.; Tang, J.Y.; Yen, C.Y.; Huang, H.W.; Wu, C.Y.; Chung, Y.A.; Wang, H.R.; Chen, I.S.; Huang, M.Y.; Chang, H.W. Antiproliferation of Cryptocarya concinna-derived cryptocaryone against oral cancer cells involving apoptosis, oxidative stress, and DNA damage. BMC Complement. Altern. Med. 2016, 16, 94. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.C.; Haung, J.W.; Chang, F.R.; Huang, K.J.; Huang, H.M.; Huang, H.W.; Chou, C.K.; Wu, Y.C.; Chang, H.W. Golden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS ONE 2013, 8, e64739. [Google Scholar] [CrossRef] [Green Version]
- Kuo, L.J.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Kasai, H.; Nishimura, S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984, 12, 2137–2145. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Ou-Yang, F.; Tsai, I.H.; Tang, J.Y.; Yen, C.Y.; Cheng, Y.B.; Farooqi, A.A.; Chen, S.R.; Yu, S.Y.; Kao, J.K.; Chang, H.W. Antiproliferation for breast cancer cells by ethyl acetate extract of Nepenthes thorellii x (ventricosa x maxima). Int. J. Mol. Sci. 2019, 20, 3238. [Google Scholar] [CrossRef] [Green Version]
- Mammadov, R.; Suleyman, B.; Altuner, D.; Demirci, E.; Cetin, N.; Yilmaz, A.; Baykal, H.; Alpcan, H.; Turumtay, E.A.; Suleyman, H. Effect of ethyl acetate extract of Usnea longissima on esophagogastric adenocarcinoma in rats. Acta Cir. Bras. 2019, 34, e201900305. [Google Scholar] [CrossRef]
- Fernandez-Moriano, C.; Gonzalez-Burgos, E.; Divakar, P.K.; Crespo, A.; Gomez-Serranillos, M.P. Evaluation of the antioxidant capacities and cytotoxic effects of ten parmeliaceae lichen species. Evid. Based Complement. Alternat. Med. 2016, 2016, 3169751. [Google Scholar] [CrossRef] [Green Version]
- Suzuki-Karasaki, Y.; Suzuki-Karasaki, M.; Uchida, M.; Ochiai, T. Depolarization controls TRAIL-sensitization and tumor-selective killing of cancer cells: Crosstalk with ROS. Front. Oncol. 2014, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [Green Version]
- Truong, T.L.; Nga, V.T.; Huy, D.T.; Chi, H.B.; Phung, N.K. A new depside from Usnea aciculifera growing in Vietnam. Nat. Prod. Commun. 2014, 9, 1179–1180. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Yoon, S.; Yang, Y.; Lee, H.B.; Oh, S.; Jeong, M.H.; Kim, J.J.; Yee, S.T.; Crisan, F.; Moon, C.; et al. Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials. PLoS ONE 2014, 9, e111575. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.-Y.; Wu, K.-H.; Wang, Y.-Y.; Farooqi, A.A.; Huang, H.-W.; Yuan, S.-S.F.; Jian, R.-I.; Tsao, L.-Y.; Chen, P.-A.; Chang, F.-R.; et al. Methanol Extract of Usnea barbata Induces Cell Killing, Apoptosis, and DNA Damage against Oral Cancer Cells through Oxidative Stress. Antioxidants 2020, 9, 694. https://doi.org/10.3390/antiox9080694
Tang J-Y, Wu K-H, Wang Y-Y, Farooqi AA, Huang H-W, Yuan S-SF, Jian R-I, Tsao L-Y, Chen P-A, Chang F-R, et al. Methanol Extract of Usnea barbata Induces Cell Killing, Apoptosis, and DNA Damage against Oral Cancer Cells through Oxidative Stress. Antioxidants. 2020; 9(8):694. https://doi.org/10.3390/antiox9080694
Chicago/Turabian StyleTang, Jen-Yang, Kuang-Han Wu, Yen-Yun Wang, Ammad Ahmad Farooqi, Hurng-Wern Huang, Shyng-Shiou F. Yuan, Ru-In Jian, Li-Yi Tsao, Po-An Chen, Fang-Rong Chang, and et al. 2020. "Methanol Extract of Usnea barbata Induces Cell Killing, Apoptosis, and DNA Damage against Oral Cancer Cells through Oxidative Stress" Antioxidants 9, no. 8: 694. https://doi.org/10.3390/antiox9080694
APA StyleTang, J.-Y., Wu, K.-H., Wang, Y.-Y., Farooqi, A. A., Huang, H.-W., Yuan, S.-S. F., Jian, R.-I., Tsao, L.-Y., Chen, P.-A., Chang, F.-R., Cheng, Y.-B., Hu, H.-C., & Chang, H.-W. (2020). Methanol Extract of Usnea barbata Induces Cell Killing, Apoptosis, and DNA Damage against Oral Cancer Cells through Oxidative Stress. Antioxidants, 9(8), 694. https://doi.org/10.3390/antiox9080694