Integrated Hypoxia Signaling and Oxidative Stress in Developmental Neurotoxicity of Benzo[a]Pyrene in Zebrafish Embryos
Abstract
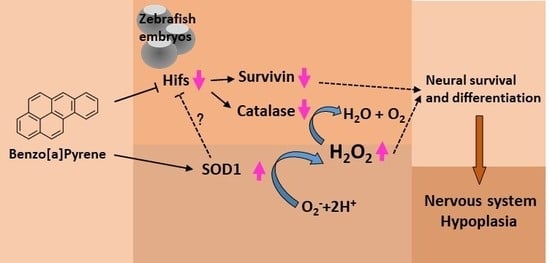
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Care and Maintenance
2.3. Benzo[a]pyrene Treatment and Embryo Processing
2.4. In Situ Hybridization Staining
2.5. Embryonic MDA and Hydrogen Peroxide Measurement
2.6. Real-Time Quantitative PCR
2.7. Statistical Tests
3. Results
3.1. Exposure of B[a]P Causes Reduced Expression of shh and islt1 in the Developing Neural System at 24 hpf
3.2. No Obvious Neural Defects Are Present in B[a]P-Exposed Embryos at 12 hpf
3.3. B[a]P Exposure Alters the Expression of Hypoxia-Related and Oxidative Stress-Related Genes
3.4. Exposure to B[a]P Increases Hydrogen Peroxide and Lipid Peroxidation
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sasai, Y.; Lu, B.; Steinbeisser, H.; De Robertis, E.M. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 1995, 376, 333–336. [Google Scholar] [CrossRef]
- Linker, C.; Stern, C.D. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development 2004, 131, 5671–5681. [Google Scholar] [CrossRef] [Green Version]
- Committee NRC. Polycyclic Aromatic Hydrocarbons: Evaluation of Sources and Effects; National Academies Press: Washington, DC, USA, 1983. [Google Scholar]
- Guo, Z.; Lin, T.; Zhang, G.; Yang, Z.; Fang, M. High-resolution depositional records of polycyclic aromatic hydrocarbons in the central continental shelf mud of the East China Sea. Environ. Sci. Technol. 2006, 40, 5304–5311. [Google Scholar] [CrossRef]
- Selkirk, J.K. Benzo[a]pyrene carcinogenesis: A biochemical selection mechanism. J. Toxicol. Environ. Health Part A 1977, 2, 1245–1258. [Google Scholar] [CrossRef]
- Lindeman, T.E.; Poirier, M.C.; Divi, R.L. The resveratrol analogue, 2, 3′, 4, 5′-tetramethoxystilbene, does not inhibit CYP gene expression, enzyme activity and benzo[a]pyrene-DNA adduct formation in MCF-7 cells exposed to benzo[a]pyrene. Mutagenesis 2011, 26, 629–635. [Google Scholar] [CrossRef]
- IARC. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 94. [Google Scholar]
- Hylland, K. Polycyclic Aromatic Hydrocarbon (PAH) Ecotoxicology in Marine Ecosystems. J. Toxicol. Environ. Health Part A 2006, 69, 109–123. [Google Scholar] [CrossRef]
- Van De Wiele, T.; Vanhaecke, L.; Boeckaert, C.; Peru, K.; Headley, J.; Verstraete, W.; Siciliano, S. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ. Health Perspect. 2005, 113, 6–10. [Google Scholar] [CrossRef]
- Wu, J.; Ramesh, A.; Nayyar, T.; Hood, D.B. Assessment of metabolites and AhR and CYP1A1 mRNA expression subsequent to prenatal exposure to inhaled benzo(a)pyrene. Int. J. Dev. Neurosci. 2003, 21, 333–346. [Google Scholar] [CrossRef]
- Davila, D.R.; Romero, D.L.; Burchiel, S.W. Human T cells are highly sensitive to suppression of mitogenesis by polycyclic aromatic hydrocarbons and this effect is differentially reversed by alpha-naphthoflavone. Toxicol. Appl. Pharmacol. 1996, 139, 333–341. [Google Scholar] [CrossRef]
- Mendola, P.; Selevan, S.G.; Gutter, S.; Rice, D. Environmental factors associated with a spectrum of neurodevelopmental deficits. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 188–197. [Google Scholar] [CrossRef]
- Min, L.; He, S.; Chen, Q.; Peng, F.; Peng, H.; Xie, M. Comparative proteomic analysis of cellular response of human airway epithelial cells (A549) to benzo(a)pyrene. Toxicol. Mech. Methods 2011, 21, 374–382. [Google Scholar] [CrossRef]
- Wolterbeek, A.P.; Schoevers, E.J.; Rutten, A.A.; Feron, V.J. A critical appraisal of intratracheal instillation of benzo[a]pyrene to Syrian golden hamsters as a model in respiratory tract carcinogenesis. Cancer Lett. 1995, 89, 107–116. [Google Scholar] [CrossRef]
- Jones, D.P. Extracellular Redox State: Refining the Definition of Oxidative Stress in Aging. Rejuvenation Res. 2006, 9, 169–181. [Google Scholar] [CrossRef]
- Castro-Obregón, S.; Covarrubias, L. Role of retinoic acid and oxidative stress in embryonic stem cell death and neuronal differentiation. FEBS Lett. 1996, 381, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Khanna, P.; Wong, B.S.E.; Heng, Z.S.L.; Subhramanyam, C.S.; Thanga, L.Z.; Tan, S.W.S.; Baeg, G.H. Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget 2017, 9, 4223–4238. [Google Scholar] [CrossRef] [Green Version]
- Guillaumet-Adkins, A.; Yañez, Y.; Peris-Diaz, M.D.; Calabria, I.; Palanca-Ballester, C.; Sandoval, J. Epigenetics and Oxidative Stress in Aging. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Maluf, S.W.; Marroni, N.P.; Heuser, V.D.; Prá, D. DNA Damage and Oxidative Stress in Human Disease. BioMed Res. Int. 2013, 2013, 1–2. [Google Scholar] [CrossRef]
- Neofytou, E.; Tzortzaki, E.G.; Chatziantoniou, A.; Siafakas, N.M. DNA Damage Due to Oxidative Stress in Chronic Obstructive Pulmonary Disease (COPD). Int. J. Mol. Sci. 2012, 13, 16853–16864. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [Green Version]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Boil. Med. 2010, 49, 1147–1151. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar]
- Chui, A.; Zhang, Q.; Dai, Q.; Shi, S.-H. Oxidative stress regulates progenitor behavior and cortical neurogenesis. Development 2020, 147, dev184150. [Google Scholar] [CrossRef]
- Maxwell, P.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Nanka, O.; Valasek, P.; Dvorakova, M.; Grim, M. Experimental hypoxia and embryonic angiogenesis. Dev. Dyn. 2006, 235, 723–733. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Tsai, M.-Y.; Tseng, W.-F.; Cheng, C.-H.; Huang, C.-R.; Wu, J.-S.; Chung, H.-Y.; Hsieh, C.-S.; Sun, C.; Hwang, S.-P.L.; et al. Integration of CNS survival and differentiation by HIF2α. Cell Death Differ. 2011, 18, 1757–1770. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B.; Schilling, T.F.; Postlethwait, J.H. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 1993, 119, 1203–1215. [Google Scholar]
- Kaji, T.; Artinger, K.B. dlx3b and dlx4b function in the development of Rohon-Beard sensory neurons and trigeminal placode in the zebrafish neurula. Dev. Boil. 2004, 276, 523–540. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, A.; Warren, J.T.; Takahashi, K.; E Schauerte, H.; Van Eeden, F.J.; Haffter, P.; Kuwada, J.Y. Role of sonic hedgehog in branchiomotor neuron induction in zebrafish. Mech. Dev. 1998, 76, 101–115. [Google Scholar] [CrossRef]
- Paffett-Lugassy, N.; Hsia, N.; Fraenkel, P.G.; Paw, B.H.; Leshinsky, I.; Barut, B.; Bahary, N.; Caro, J.; Handin, R.; Zon, L.I. Functional conservation of erythropoietin signaling in zebrafish. Blood 2007, 110, 2718–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delvaeye, M.; De Vriese, A.; Zwerts, F.; Betz, I.; Moons, M.; Autiero, M.; Conway, E.M. Role of the 2 zebrafish survivin genes in vasculo-angiogenesis, neurogenesis, cardiogenesis and hematopoiesis. BMC Dev. Boil. 2009, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.-Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Wikenheiser, J.; Karunamuni, G.; Sloter, E.; Walker, M.K.; Roy, D.; Wilson, D.L.; Watanabe, M. Altering HIF-1α through 2, 3, 7, 8-Tetrachlorodibenzo-p-Dioxin (TCDD) Exposure Affects Coronary Vessel Development. Cardiovasc. Toxicol. 2012, 13, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Tomita, S.; Ueno, M.; Sakamoto, M.; Kitahama, Y.; Ueki, M.; Maekawa, N.; Sakamoto, H.; Gassmann, M.; Kageyama, R.; Ueda, N.; et al. Defective Brain Development in Mice Lacking the Hif-1α Gene in Neural Cells. Mol. Cell. Boil. 2003, 23, 6739–6749. [Google Scholar] [CrossRef] [Green Version]
- Alnaeeli, M.; Wang, L.; Piknova, B.; Rogers, H.; Li, X.; Noguchi, C.T. Erythropoietin in Brain Development and Beyond. Anat. Res. Int. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, J.A.; Clinkenbeard, E.L. Regulation of Fibroblast Growth Factor 23 by Iron, EPO, and HIF. Curr. Mol. Boil. Rep. 2019, 5, 8–17. [Google Scholar] [CrossRef]
- Adelman, D.M.; Maltepe, E.; Simon, M.C. HIF-1 is Essential for Multilineage Hematopoiesis in the Embryo. Retinal Degener. Dis. 2002, 475, 275–284. [Google Scholar]
- Zhou, D.; Han, S.; Yan, T.; Long, C.; Xu, J.; Zheng, P.; Chen, Z.; Jia, G. Toxicity of Titanium Dioxide Nanoparticles Induced by Reactive Oxygen Species. React. Oxyg. Species 2019, 8, 267–275. [Google Scholar]
- Parke, D.V.; Sapota, A. Chemical toxicity and reactive oxygen species. Int. J. Occup. Med. Environ. Health 1996, 9, 331–340. [Google Scholar]
- Buonocore, G.; Perrone, S.; Tataranno, M.L. Oxygen toxicity: Chemistry and biology of reactive oxygen species. Semin. Fetal Neonatal Med. 2010, 15, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Sarubbi, F.; Rossetti, C.; Grazioli, G.; Di Meo, G.P.; Iannuzzi, L. Effect of dioxin exposure on several indices of blood redox status in lactating buffalo cows. J. Dairy Res. 2011, 78, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Sokol, R.J.; Devereaux, M.W.; Traber, M.G.; Shikes, R.H. Copper toxicity and lipid peroxidation in isolated rat hepatocytes: Effect of vitamin E. Pediatr. Res. 1989, 25, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurman, R.G.; Oshino, N.; Chance, B. The Role of Hydrogen Peroxide Production and Catalase in Hepatic Ethanol Metabolism. Retinal Degener. Dis. 1975, 59, 163–183. [Google Scholar]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anti-Cancer Agents Med. Chem. 2011, 11, 341–346. [Google Scholar] [CrossRef]
- Blaess, S.; Szabo, N.; Haddad-Tóvolli, R.; Zhou, X.; Alvarez-Bolado, G. Sonic hedgehog signaling in the development of the mouse hypothalamus. Front. Neuroanat. 2015, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Corman, T.S.; Bergendahl, S.E.; Epstein, D.J. Distinct temporal requirements for Sonic hedgehog signaling in development of the tuberal hypothalamus. Development 2018, 145, dev167379. [Google Scholar] [CrossRef] [Green Version]
- Scholpp, S.; Lumsden, A. Building a bridal chamber: Development of the thalamus. Trends Neurosci. 2010, 33, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Samet, J.M.; Wages, P.A. Oxidative Stress from Environmental Exposures. Curr. Opin. Toxicol. 2018, 7, 60–66. [Google Scholar] [CrossRef]
- Cervellati, F.; Cervellati, C.; Romani, A.; Cremonini, E.; Sticozzi, C.; Belmonte, G.; Pessina, F.; Valacchi, G. Hypoxia induces cell damage via oxidative stress in retinal epithelial cells. Free Radic. Res. 2014, 48, 303–312. [Google Scholar] [CrossRef]
- Al-Gubory, K.H. Multiple exposures to environmental pollutants and oxidative stress: Is there a sex specific risk of developmental complications for fetuses? Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 351–364. [Google Scholar]
- Rossignol, D.A.; Frye, R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Adida, C.; Crotty, P.L.; McGrath, J.; Berrebi, D.; Diebold, J.; Altieri, D.C. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am. J. Pathol. 1998, 152, 43–49. [Google Scholar]
- Jiang, Y.; De Bruin, A.; Caldas, H.; Fangusaro, J.; Hayes, J.; Conway, E.M.; Robinson, M.L.; Altura, R.A. Essential Role for Survivin in Early Brain Development. J. Neurosci. 2005, 25, 6962–6970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharova, E.T.; Sokolov, A.V.; Pavlichenko, N.; Kostevich, V.A.; Abdurasulova, I.N.; Chechushkov, A.V.; Voynova, I.V.; Elizarova, A.Y.; Kolmakov, N.N.; Bass, M.G.; et al. Erythropoietin and Nrf2: Key factors in the neuroprotection provided by apo-lactoferrin. BioMetals 2018, 31, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Digicaylioglu, M.; Lipton, S.A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature 2001, 412, 641–647. [Google Scholar] [CrossRef]
- Yu, X.; Shacka, J.J.; Eells, J.B.; Suarez-Quian, C.; Przygodzki, R.M.; Beleslin-Cokic, B.; Lin, C.-S.; Nikodem, V.M.; Hempstead, B.; Flanders, K.C.; et al. Erythropoietin receptor signalling is required for normal brain development. Development 2002, 129, 505–516. [Google Scholar]
- Wakhloo, D.; Scharkowski, F.; Curto, Y.; Butt, U.J.; Bansal, V.; Steixner-Kumar, A.A.; Wüstefeld, L.; Rajput, A.; Arinrad, S.; Zillmann, M.R.; et al. Functional hypoxia drives neuroplasticity and neurogenesis via brain erythropoietin. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkowsky, J.L.; Son, J.-H. Hypoxia and connectivity in the developing vertebrate nervous system. Dis. Model. Mech. 2018, 11, dmm037127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scortegagna, M.; Ding, K.; Oktay, Y.; Gaur, A.; Thurmond, F.; Yan, L.J.; Marck, B.T.; Matsumoto, A.M.; Shelton, J.M.; Richardson, J.A.; et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/-mice. Nat. Genet. 2003, 35, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Rieger, S.; Sagasti, A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS ONE Biol. 2011, 9, e1000621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen Peroxide Sensing and Signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rampon, C.; Volovitch, M.; Joliot, A.; Vriz, S. Hydrogen Peroxide and Redox Regulation of Developments. Antioxidants 2018, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Tomita, K.; Takashi, Y.; Ouchi, Y.; Kuwahara, Y.; Igarashi, K.; Nagasawa, T.; Nabika, H.; Kurimasa, A.; Fukumoto, M.; Nishitani, Y.; et al. Lipid peroxidation increases hydrogen peroxide permeability leading to cell death in cancer cell lines that lack mtDNA. Cancer Sci. 2019, 110, 2856–2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellogg, E.W.; Fridovich, I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J. Boil. Chem. 1975, 250, 8812–8817. [Google Scholar]
- McNeill-Blue, C.; Wetmore, B.A.; Sanchez, J.F.; Freed, W.J.; Merrick, B.A. Apoptosis mediated by p53 in rat neural AF5 cells following treatment with hydrogen peroxide and staurosporine. Brain Res. 2006, 1112, 1–15. [Google Scholar] [CrossRef]
- Reis, A.C.; Alessandri, A.L.; Athayde, R.M.; Perez, D.A.; Vago, J.P.; Ávila, T.V.; Ferreira, T.P.T.; De Arantes, A.C.S.; Coutinho, D.; Rachid, M.A.; et al. Induction of eosinophil apoptosis by hydrogen peroxide promotes the resolution of allergic inflammation. Cell Death Dis. 2015, 6, e1632. [Google Scholar] [CrossRef]
- Wittmann, C.; Chockley, P.; Singh, S.K.; Pase, L.; Lieschke, G.J.; Grabher, C. Hydrogen Peroxide in Inflammation: Messenger, Guide, and Assassin. Adv. Hematol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Qamar, W.; Khan, A.Q.; Khan, R.; Lateef, A.; Tahir, M.; Rehman, M.U.; Ali, F.; Sultana, S. Benzo(a)pyrene-induced pulmonary inflammation, edema, surfactant dysfunction, and injuries in rats: Alleviation by farnesol. Exp. Lung Res. 2011, 38, 19–27. [Google Scholar] [CrossRef]
- Das, S.K.; Patri, M. Neuropeptide Y expression confers benzo[a]pyrene induced anxiolytic like behavioral response during early adolescence period of male Wistar rats. Neuropeptides 2017, 61, 23–30. [Google Scholar] [CrossRef]
- Mohanty, R.; Das, S.K.; Singh, N.R.; Patri, M. Withania somnifera Leaf Extract Ameliorates Benzo[a]pyrene-Induced Behavioral and Neuromorphological Alterations by Improving Brain Antioxidant Status in Zebrafish (Danio rerio). Zebrafish 2016, 13, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Das, S.K.; Patri, M. Neonatal Benzo[a]pyrene Exposure Induces Oxidative Stress and DNA Damage Causing Neurobehavioural Changes during the Early Adolescence Period in Rats. Dev. Neurosci. 2016, 38, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Seth, P.K.; Mukhtar, H. Distribution of benzo(a)pyrene in discrete regions of rat brain. Bull. Environ. Contam. Toxicol. 1985, 35, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Grova, N.; Schroeder, H.; Farinelle, S.; Prodhomme, E.; Valley, A.; Muller, C.P. Sub-acute administration of benzo[a]pyrene (B[a]P) reduces anxiety-related behaviour in adult mice and modulates regional expression of N-methyl-d-aspartate (NMDA) receptors genes in relevant brain regions. Chemosphere 2008, 73, S295–S302. [Google Scholar] [CrossRef]
- Saunders, C.R.; Das, S.K.; Ramesh, A.; Shockley, D.C.; Mukherjee, S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: Role of oxidative stress. J. Appl. Toxicol. 2006, 26, 427–438. [Google Scholar] [CrossRef]
- Cheng, S.-Q.; Xia, Y.-Y.; He, J.-L.; Liu, X.-Q.; Chen, X.; Ding, Y.-B.; Wang, Y.-X.; Peng, B.; Tu, B.-J. Neurotoxic effect of subacute benzo(a)pyrene exposure on gene and protein expression in Sprague-Dawley rats. Environ. Toxicol. Pharmacol. 2013, 36, 648–658. [Google Scholar] [CrossRef]
- Dayal, H.; Gupta, S.; Trieff, N.; Maierson, D.; Reich, D. Symptom Clusters in a Community with Chronic Exposure to Chemicals in Two Superfund Sites. Arch. Environ. Health Int. J. 1995, 50, 108–111. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, F.; Zheng, J.; Li, S.; Qiang, M. Chronic Administration of Benzo(a)pyrene Induces Memory Impairment and Anxiety-Like Behavior and Increases of NR2B DNA Methylation. PLoS ONE 2016, 11, e0149574. [Google Scholar] [CrossRef]
- Youbin, Q.; Chengzhi, C.; Yan, T.; Xuejun, J.; Chongying, Q.; Bin, P.; Tu, B. The synergistic effect of benzo[a]pyrene and lead on learning and memory of mice. Toxicol. Ind. Health 2012, 29, 387–395. [Google Scholar] [CrossRef]
- Lin, S.; Ren, A.; Wang, L.; Huang, Y.; Wang, Y.; Wang, C.; Greene, N.D. Oxidative Stress and Apoptosis in Benzo[a]pyrene-Induced Neural Tube Defects. Free Radic. Boil. Med. 2018, 116, 149–158. [Google Scholar] [CrossRef]
- Mohanty, R.; Das, S.K.; Patri, M. Modulation of Benzo[a]Pyrene Induced Anxiolytic-Like Behavior by Retinoic Acid in Zebrafish: Involvement of Oxidative Stress and Antioxidant Defense System. Neurotox. Res. 2017, 31, 493–504. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Wu, C.-Y.; Hu, C.-H.; Pai, T.-W.; Chen, Y.-R.; Wang, W.-D. Integrated Hypoxia Signaling and Oxidative Stress in Developmental Neurotoxicity of Benzo[a]Pyrene in Zebrafish Embryos. Antioxidants 2020, 9, 731. https://doi.org/10.3390/antiox9080731
Lin Y-C, Wu C-Y, Hu C-H, Pai T-W, Chen Y-R, Wang W-D. Integrated Hypoxia Signaling and Oxidative Stress in Developmental Neurotoxicity of Benzo[a]Pyrene in Zebrafish Embryos. Antioxidants. 2020; 9(8):731. https://doi.org/10.3390/antiox9080731
Chicago/Turabian StyleLin, Yi-Chen, Chang-Yi Wu, Chin-Hwa Hu, Tun-Wen Pai, Yet-Ran Chen, and Wen-Der Wang. 2020. "Integrated Hypoxia Signaling and Oxidative Stress in Developmental Neurotoxicity of Benzo[a]Pyrene in Zebrafish Embryos" Antioxidants 9, no. 8: 731. https://doi.org/10.3390/antiox9080731
APA StyleLin, Y. -C., Wu, C. -Y., Hu, C. -H., Pai, T. -W., Chen, Y. -R., & Wang, W. -D. (2020). Integrated Hypoxia Signaling and Oxidative Stress in Developmental Neurotoxicity of Benzo[a]Pyrene in Zebrafish Embryos. Antioxidants, 9(8), 731. https://doi.org/10.3390/antiox9080731