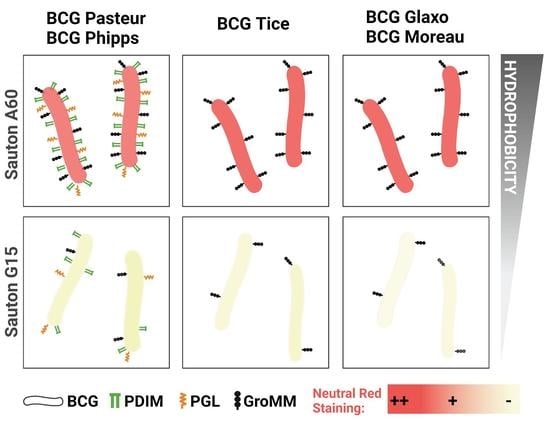
BCG Substrains Change Their Outermost Surface as a Function of Growth Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Extraction of Superficial Non-Covalently Linked Lipids
2.3. Neutral Red Staining
2.4. BCG Hydrophobicity by Hexadecane-Aqueous Buffer Partitioning
2.5. BCG RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Assay
2.6. Statistical Analysis
3. Results
3.1. The Outermost Lipids and Hydrophobicity of the BCG Cell Wall Depend on the Growth Conditions
3.2. Culture Media Composition Influences BCG Neutral Red Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brosch, R.; Gordon, S.V.; Garnier, T.; Eiglmeier, K.; Frigui, W.; Valenti, P.; Garcia-pelayo, C.; Inwald, J.K.; Dos Santos, S.; Hewinson, R.G.; et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl. Acad. Sci. USA 2007, 104, 5596–5601. [Google Scholar] [CrossRef] [Green Version]
- Prados-Rosales, R.; Carreño, L.J.; Weinrick, B.; Batista-Gonzalez, A.; Glatman-Freedman, A.; Xu, J.; Chan, J.; Jacobs, W.R.; Porcelli, S.A.; Casadevall, A. The type of growth medium affects the presence of a mycobacterial capsule and is associated with differences in protective efficacy of BCG vaccination against Mycobacterium tuberculosis. J. Infect. Dis. 2016, 214, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H. High-viability lyophilized Bacille Calmette-Guerin vaccine produced by deep-culture technique. Appl. Environ. Microbiol. 1977, 34, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Expert Committee on Biological Standardization Recommendations to assure the quality, safety and efficacy of BCG vaccines. World Health Organ. Tech. Rep. Ser. 2013, 979, 1–366. [Google Scholar]
- Noguera-Ortega, E.; Guallar-Garrido, S.; Julián, E. Mycobacteria-based vaccines as immunotherapy for non-urological cancers. Cancers 2020, 12, 1802. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Netea, M.G. BCG-induced trained immunity: Can it offer protection against COVID-19? Nat. Rev. Immunol. 2020, 20, 335–337. [Google Scholar] [CrossRef]
- Tran, V.; Ahn, S.K.; Ng, M.; Li, M.; Liu, J. Loss of Lipid Virulence Factors Reduces the Efficacy of the BCG Vaccine. Sci. Rep. 2016, 6, 29076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secanella-Fandos, S.; Luquin, M.; Julián, E. Connaught and russian strains showed the highest direct antitumor effects of different bacillus calmette-guérin substrains. J. Urol. 2013, 189, 711–718. [Google Scholar] [CrossRef]
- Guallar-Garrido, S.; Campo-Pérez, V.; Sánchez-Chardi, A.; Luquin, M.; Julián, E. Each Mycobacterium Requires a Specific Culture Medium Composition for Triggering an Optimized Immunomodulatory and Antitumoral Effect. Microorganisms 2020, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Guallar-Garrido, S.; Luquin, M.; Julián, E. Analysis of the Lipid Composition of Mycobacteria by Thin Layer Chromatography. J. Vis. Exp. 2021, 170, e62368. [Google Scholar] [CrossRef] [PubMed]
- Andreu, N.; Soto, C.Y.; Roca, I.; Martín, C.; Gibert, I. Mycobacterium smegmatis displays the Mycobacterium tuberculosis virulence-related neutral red character when expressing the Rv0577 gene. FEMS Microbiol. Lett. 2004, 231, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 2–33. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Hill-Cawthorne, G.A.; Otto, T.D.; Coll, F.; Guerra-Assunção, J.A.; Gao, G.; Naeem, R.; Ansari, H.; Malas, T.B.; Adroub, S.A.; et al. Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci. Rep. 2015, 5, 15443. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, A.M.; Behr, M.A. Evolution and Strain Variation in BCG. Adv. Exp. Med. Biol. 2017, 1019, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.S.; Tran, V.; Wu, Z.; Yu, X.; Alexander, D.C.; Gao, G.F.; Zhu, B.; Liu, J. Novel genome polymorphisms in BCG vaccine strains and impact on efficacy. BMC Genom. 2008, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Naka, T.; Maeda, S.; Niki, M.; Ohara, N.; Yamamoto, S.; Yano, I.; Maeyama, J.I.; Ogura, H.; Kobayashi, K.; Fujiwara, N. Lipid phenotype of two distinct subpopulations of Mycobacterium bovis bacillus calmette-guérin Tokyo 172 substrain. J. Biol. Chem. 2011, 286, 44153–44161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelidou, A.; Conti, M.G.; Diray-Arce, J.; Benn, C.S.; Shann, F.; Netea, M.G.; Liu, M.; Potluri, L.P.; Sanchez-Schmitz, G.; Husson, R.; et al. Licensed Bacille Calmette-Guérin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine 2020, 38, 2229–2240. [Google Scholar] [CrossRef]
- Domenech, P.; Reed, M.B. Rapid and spontaneous loss of phthiocerol dimycocerosate (PDIM) from Mycobacterium tuberculosis grown in vitro: Implications for virulence studies. Microbiology 2009, 155, 3532–3543. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.A.S.; Rosenkrands, I.; Olsen, A.W.; Nordly, P.; Christensen, D.; Lang, R.; Kirschning, C.; Gomes, J.M.; Bhowruth, V.; Minnikin, D.E.; et al. Novel Generation Mycobacterial Adjuvant Based on Liposome-Encapsulated Monomycoloyl Glycerol from Mycobacterium bovis Bacillus Calmette-Guerin. J. Immunol. 2009, 183, 2294–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, Y.; Matsunaga, I.; Komori, T.; Urakawa, T.; Nakamura, T.; Fujiwara, N.; Hiromatsu, K.; Harashima, H.; Sugita, M. Glycerol monomycolate, a latent tuberculosis-associated mycobacterial lipid, induces eosinophilic hypersensitivity responses in guinea pigs. Biochem. Biophys. Res. Commun. 2011, 409, 304–307. [Google Scholar] [CrossRef]
- Venkataswamy, M.M.; Goldberg, M.F.; Baena, A.; Chan, J.; Jacobs, W.R.; Porcelli, S.A. In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG. Vaccine 2012, 30, 1038–1049. [Google Scholar] [CrossRef] [Green Version]
- Guallar-Garrido, S.; Campo-Pérez, V.; Pérez-Trujillo, M.; Cabrera, C.; Senserrich, J.; Sánchez-Chardi, A.; Rabanal, R.M.; Gómez-Mora, E.; Noguera-Ortega, E.; Luquin, M.; et al. Mycobacterial surface characters remodeled by growth conditions drive different tumor-infiltrating cells and IFN-/IL-17 release in bladder cancer treatment. Oncoimmunology 2021. submitted. [Google Scholar]
- Guerra-Maupome, M.; Vang, D.X.; McGill, J.L. Aerosol vaccination with Bacille Calmette-Guerin induces a trained innate immune phenotype in calves. PLoS ONE 2019, 14, e0212751. [Google Scholar] [CrossRef] [Green Version]
- Cardona, P.J.; Soto, C.Y.; Martín, C.; Giquel, B.; Agustí, G.; Guirado, E.; Sirakova, T.; Kolattukudy, P.; Julián, E.; Luquin, M. Neutral-red reaction is related to virulence and cell wall methyl-branched lipids in Mycobacterium tuberculosis. Microbes Infect. 2006, 8, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Huard, R.C.; Chitale, S.; Leung, M.; Lazzarini, L.C.O.; Zhu, H.; Shashkina, E.; Laal, S.; Conde, M.B.; Kritski, A.L.; Belisle, J.T.; et al. The Mycobacterium tuberculosis Complex-Restricted Gene cfp32 Encodes an Expressed Protein That Is Detectable in Tuberculosis Patients and Is Positively Correlated with Pulmonary Interleukin-10. Infect. Immun. 2003, 71, 6871–6883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethe, K.; Sequeira, P.C.; Agarwalla, S.; Rhee, K.; Kuhen, K.; Phong, W.Y.; Patel, V.; Beer, D.; Walker, J.R.; Duraiswamy, J.; et al. A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat. Commun. 2010, 1, 57. [Google Scholar] [CrossRef]
- Buchko, G.W.; Echols, N.; Flynn, E.M.; Ng, H.L.; Stephenson, S.; Kim, H.B.; Myler, P.J.; Terwilliger, T.C.; Alber, T.; Kim, C.Y. Structural and Biophysical Characterization of the Mycobacterium tuberculosis Protein Rv0577, a Protein Associated with Neutral Red Staining of Virulent Tuberculosis Strains and Homologue of the Streptomyces coelicolor Protein KbpA. Biochemistry 2017, 56, 4015–4027. [Google Scholar] [CrossRef]
- Byun, E.; Kim, W.S.; Kim, J.; Jung, I.D.; Park, Y.; Kim, H.; Cho, S.; Shin, S.J. Mycobacterium tuberculosis Rv0577, a novel TLR2 agonist, induces maturation of dendritic cells and drives Th1 immune response. FASEB J. 2012, 26, 2695–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Culture Media | BCG Pasteur | BCG Phipps | BCG Tice | BCG Glaxo | BCG Moreau | |
|---|---|---|---|---|---|---|
| PDIM | A60 | +++ | ++ | - | - | - |
| G15 | - | - | - | - | - | |
| AG | A60 | +++ | +++ | - | - | + |
| G15 | + | - | - | - | + | |
| PGL | A60 | ++ | + | - | - | - |
| G15 | - | - | - | - | - | |
| GroMM | A60 | +++ | +++ | ++ | ++ | ++ |
| G15 | - | - | + | + | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guallar-Garrido, S.; Almiñana-Rapún, F.; Campo-Pérez, V.; Torrents, E.; Luquin, M.; Julián, E. BCG Substrains Change Their Outermost Surface as a Function of Growth Media. Vaccines 2022, 10, 40. https://doi.org/10.3390/vaccines10010040
Guallar-Garrido S, Almiñana-Rapún F, Campo-Pérez V, Torrents E, Luquin M, Julián E. BCG Substrains Change Their Outermost Surface as a Function of Growth Media. Vaccines. 2022; 10(1):40. https://doi.org/10.3390/vaccines10010040
Chicago/Turabian StyleGuallar-Garrido, Sandra, Farners Almiñana-Rapún, Víctor Campo-Pérez, Eduard Torrents, Marina Luquin, and Esther Julián. 2022. "BCG Substrains Change Their Outermost Surface as a Function of Growth Media" Vaccines 10, no. 1: 40. https://doi.org/10.3390/vaccines10010040
APA StyleGuallar-Garrido, S., Almiñana-Rapún, F., Campo-Pérez, V., Torrents, E., Luquin, M., & Julián, E. (2022). BCG Substrains Change Their Outermost Surface as a Function of Growth Media. Vaccines, 10(1), 40. https://doi.org/10.3390/vaccines10010040