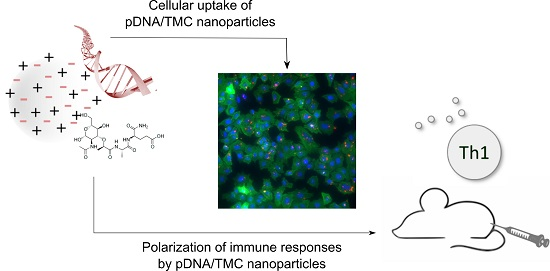
Ag85A DNA Vaccine Delivery by Nanoparticles: Influence of the Formulation Characteristics on Immune Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Preparation
2.3. Size and Zeta Potential
2.4. Loading Efficiency
2.5. pDNA Release Profile
2.6. Nanoparticle Uptake In Vitro
2.7. Immunization of Mice with pDNA-Loaded Nanoparticles
2.8. Antibody ELISA
2.9. IFN-γ ELISPOT
2.10. Statistical Methods
3. Results
3.1. Nanoparticle Characterization
3.2. Kinetics of pDNA Release
3.3. Cellular Uptake
3.4. Immunogenicity of Different pDNA/TMC Nanoparticle Ratios
3.5. Impact of MDP on the pDNA/TMC-NPs-0.7 Formulation
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoft, D.F.; Kemp, E.B.; Marinaro, M.; Cruz, O.; Kiyono, H.; McGhee, J.R.; Belisle, J.T.; Milligan, T.W.; Miller, J.P.; Belshe, R.B. A double-blind, placebo-controlled study of mycobacterium-specific human immune responses induced by intradermal bacille calmette-guérin vaccination. J. Lab. Clin. Med. 1999, 134, 244–252. [Google Scholar] [CrossRef]
- Lindblad, E.B.; Elhay, M.J.; Silva, R.; Appelberg, R.; Andersen, P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 1997, 65, 623–629. [Google Scholar] [PubMed]
- Tameris, M.; Geldenhuys, H.; Luabeya, A.K.; Smit, E.; Hughes, J.E.; Vermaak, S.; Hanekom, W.A.; Hatherill, M.; Mahomed, H.; McShane, H. The candidate TB vaccine, MVA85A, induces highly durable Th1 responses. PLoS ONE 2014, 9, e87340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingolotti, M.; Kawalekar, O.; Shedlock, D.J.; Muthumani, K.; Weiner, D.B. DNA vaccines for targeting bacterial infections. Expert Rev. Vaccines 2010, 9, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.-M.; Jo, E.-K. Host immune responses to mycobacterial antigens and their implications for the development of a vaccine to control tuberculosis. Clin. Exp. Vaccine Res. 2014, 3, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Huygen, K.; Denis, O.; Montgomery, D.L.; Yawman, A.M.; Deck, R.R.; DeWitt, C.M.; Orme, I.M.; Baldwin, S.; D’Souza, C.; Drowart, A. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 1996, 2, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.; Denis-Mize, K.; Woo, C.; Goldbeck, C.; Selby, M.J.; Chen, M.; Otten, G.R.; Ulmer, J.B.; Donnelly, J.J.; Ott, G. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 2000, 165, 2850–2858. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Simbiri, K.A.; Sin, J.I.; Dang, K.; Oh, J.; Dentchev, T.; Lee, D.; Nottingham, L.K.; Chalian, A.A.; Mccallus, D. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J. Interferon Cytokine Res. 1999, 19, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Amidi, M.; Romeijn, S.G.; Borchard, G.; Junginger, H.E.; Hennink, W.E.; Jiskoot, W. Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J. Control. Release 2006, 111, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.M.; Verhoef, J.C.; Romeijn, S.G.; Nagelkerke, J.F.; Merkus, F.W.H.M.; Junginger, H.E. Effects of N-trimethyl chitosan chloride, a novel absorption enhancer, on Caco-2 intestinal epithelia and the ciliary beat frequency of chicken embryo trachea. Int. J. Pharm. 1999, 185, 73–82. [Google Scholar] [CrossRef]
- Slütter, B.; Plapied, L.; Fievez, V.; Alonso Sande, M.; des Rieux, A.; Schneider, Y.-J.; van Riet, E.; Jiskoot, W.; Préat, V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release 2009, 138, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.; Florea, B.; Geldof, M.; Junginger, H.; Borchard, G. Quaternized chitosan oligomers as novel gene delivery vectors in epithelial cell lines. Biomaterials 2002, 23, 153–159. [Google Scholar] [CrossRef]
- Bomford, R.; Stapleton, M.; Winsor, S.; McKnight, A.; Andronova, T. The control of the antibody isotype response to recombinant human immunodeficiency virus gp120 antigen by adjuvants. AIDS Res. Hum. Retrovir. 1992, 8, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Lemesre, J.-L.; Holzmuller, P.; Gonçalves, R.B.; Bourdoiseau, G.; Hugnet, C.; Cavaleyra, M.; Papierok, G. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of france: Double-blind randomised efficacy field trial. Vaccine 2007, 25, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Pye, D.; Vandenberg, K.L.; Dyer, S.L.; Irving, D.O.; Goss, N.H.; Woodrow, G.C.; Saul, A.; Alving, C.R.; Richards, R.L.; Ballou, W.R.; et al. Selection of an adjuvant for vaccination with the malaria antigen, MSA-2. Vaccine 1997, 15, 1017–1023. [Google Scholar] [CrossRef]
- Abbott, D.W.; Yang, Y.; Hutti, J.E.; Madhavarapu, S.; Kelliher, M.A.; Cantley, L.C. Coordinated regulation of toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Mol. Cell. Biol. 2007, 27, 6012–6025. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.C.; Mills, K.H. TLR, NLR agonists, and other immune modulators as infectious disease vaccine adjuvants. Curr. Infect. Dis. Rep. 2010, 12, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Heuking, S.; Adam-Malpel, S.; Sublet, E.; Iannitelli, A.; Stefano, A.d.; Borchard, G. Stimulation of human macrophages (Thp-1) using toll-like receptor-2 (TLR-2) agonist decorated nanocarriers. J. Drug Target. 2009, 17, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.; di Francesco, T.; Falson, F.; Rousselle, P.; Jordan, O.; Borchard, G. Preparation and evaluation of nanoparticles for directed tissue engineering. Int. J. Pharm. 2012, 439, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Jain, P.; Bettahi, I.; Pal, S.; Tifrea, D.; Luis, M. A TLR2 agonist is a more effective adjuvant for a chlamydia major outer membrane protein vaccine than ligands to other TLR and NOD receptors. Vaccine 2011, 29, 6641–6649. [Google Scholar] [CrossRef] [PubMed]
- Moschos, S.A.; Bramwell, V.W.; Somavarapu, S.; Alpar, H.O. Comparative immunomodulatory properties of a chitosan-MDP adjuvant combination following intranasal or intramuscular immunisation. Vaccine 2005, 23, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Poecheim, J.; Barnier Quer, C.; Heuking, S.; Brunner, L.; Collin, N.; Borchard, G. Nanocarriers for DNA vaccines: Co-delivery of TLR-9 and NLR-2 leads to synergistic enhancement of proinflammatory cytokine release. Nanomaterials 2015, 5, 2317–2334. [Google Scholar] [CrossRef]
- Limbach, L.K.; Li, Y.; Grass, R.N.; Brunner, T.J.; Hintermann, M.A.; Muller, M.; Gunther, D.; Stark, W.J. Oxide nanoparticle uptake in human lung fibroblasts: Effects of particle size, agglomeration, and diffusion at low concentrations. Environ. Sci. Technol. 2005, 39, 9370–9376. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Patel, P.C.; Millstone, J.E.; Rosi, N.L.; Mirkin, C.A. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007, 7, 3818–3821. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Babensee, J.E. Interaction of dendritic cells with biomaterials. Semin. Immunol. 2008, 20, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Slütter, B.; van Riet, E.; Kruithof, A.C.; Ding, Z.; Kersten, G.F.A.; Jiskoot, W.; Bouwstra, J.A. Efficient induction of immune responses through intradermal vaccination with N-trimethyl chitosan containing antigen formulations. J. Control. Release 2010, 142, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Mumper, R.J. Microparticles and nanoparticles as delivery systems for DNA vaccines. Crit. Rev. Ther. Drug Carr. Syst. 2003. [Google Scholar] [CrossRef]
- Tada, H.; Aiba, S.; Shibata, K.-I.; Ohteki, T.; Takada, H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect. Immun. 2005, 73, 7967–7976. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Sugawara, Y.; Kurata, S.; Fujimoto, Y.; Fukase, K.; Kusumoto, S.; Satta, Y.; Sasano, T.; Sugawara, S.; Takada, H. Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, Nod1 and Nod2 in human oral epithelial cells. Cell. Microbiol. 2005, 7, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, D.A.; Ghosh, S.; Butler, M.; Hunt, K.; Foxwell, B.M.J.; Mengin-Lecreulx, D.; Playford, R.J. Synergistic enhancement of toll-like receptor responses by nod1 activation. Eur. J. Immunol. 2005, 35, 2471–2476. [Google Scholar] [CrossRef] [PubMed]




| Sample Name | Fixed TMC-NP Concentration | Fixed pDNA Concentration | ||||
|---|---|---|---|---|---|---|
| Z-av (nm) | PDI | ζ (mV) | Z-av (nm) | PDI | ζ (mV) | |
| TMC-NPs unloaded | 527 ± 17 | 0.3 | 10 ± 1 | |||
| pDNA/TMC-NPs-0.07 | 684 ± 107 | 0.3 | 2 ± 0.4 | 712 ± 58 | 0.3 | 1 ± 1 |
| pDNA/TMC-0.7 | 406 ± 3 | 0.3 | −24 ± 2 | 436 ± 14 | 0.3 | −23 ± 3 |
| pDNA/TMC-3 | 485 ± 8 | 0.4 | −28 ± 2 | 401 ± 20 | 0.3 | −30 ± 3 |
| pDNA/TMC-7 | 652 ± 26 | 0.4 | −36 ± 1 | 519 ± 8 | 0.4 | −22 ± 4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poecheim, J.; Barnier-Quer, C.; Collin, N.; Borchard, G. Ag85A DNA Vaccine Delivery by Nanoparticles: Influence of the Formulation Characteristics on Immune Responses. Vaccines 2016, 4, 32. https://doi.org/10.3390/vaccines4030032
Poecheim J, Barnier-Quer C, Collin N, Borchard G. Ag85A DNA Vaccine Delivery by Nanoparticles: Influence of the Formulation Characteristics on Immune Responses. Vaccines. 2016; 4(3):32. https://doi.org/10.3390/vaccines4030032
Chicago/Turabian StylePoecheim, Johanna, Christophe Barnier-Quer, Nicolas Collin, and Gerrit Borchard. 2016. "Ag85A DNA Vaccine Delivery by Nanoparticles: Influence of the Formulation Characteristics on Immune Responses" Vaccines 4, no. 3: 32. https://doi.org/10.3390/vaccines4030032
APA StylePoecheim, J., Barnier-Quer, C., Collin, N., & Borchard, G. (2016). Ag85A DNA Vaccine Delivery by Nanoparticles: Influence of the Formulation Characteristics on Immune Responses. Vaccines, 4(3), 32. https://doi.org/10.3390/vaccines4030032