Subcutaneous Immunization of Leishmania HSP70-II Null Mutant Line Reduces the Severity of the Experimental Visceral Leishmaniasis in BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Parasites
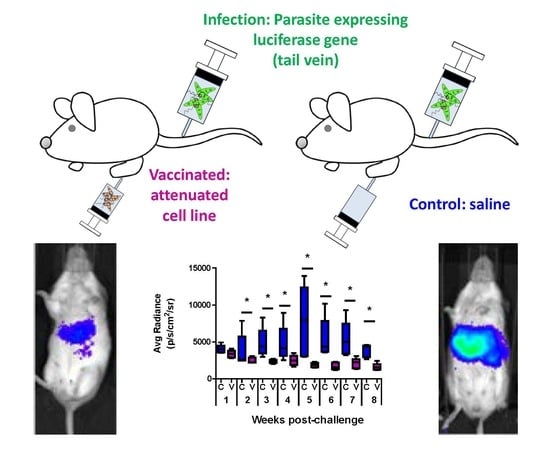
2.2. Follow-up of in Vivo Infections by BLI
2.3. Quantification of Parasites in Liver, Spleen, and Bone Marrow (BM) by Limiting Dilution
2.4. Serum Preparation and Analysis of the Humoral Responses
2.5. Determination of Cytokine Concentrations in Culture Supernatants
2.6. Cell Cytometry Analyses
2.7. Statistical Analysis
3. Results
3.1. Comparative Analysis of Evolution of Leishmania Infection in Vaccinated and Control Mice
3.2. Vaccinated Animals Showed an Earlier Humoral Response against Leishmanial Antigens after Infective Challenge
3.3. Vaccination with the Attenuated LiΔHSP70-II Line Anticipates the Parasite-Specific Cellular Immune Response after the Infective Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Goto, Y.; Ghosh, P.; Mondal, D. Impact of sequelae of visceral leishmaniasis and their contribution to ongoing transmission of Leishmania donovani. Pathog. Dis. 2019, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckingham-Jeffery, E.; Hill, E.M.; Datta, S.; Dilger, E.; Courtenay, O. Spatio-temporal modelling of Leishmania infantum infection among domestic dogs: A simulation study and sensitivity analysis applied to rural Brazil. Parasit. Vectors 2019, 12, 215. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, V.M.; da Silva, S.M.; Menz, I.; Tabanez, P.; Nogueira Fdos, S.; Werkhauser, M.; da Fonseca, A.L.; Dantas-Torres, F. Control of visceral leishmaniasis in Brazil: Recommendations from Brasileish. Parasit. Vectors 2013, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Solano-Gallego, L.; Miro, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G.; The LeishVet, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [Green Version]
- Miro, G.; Petersen, C.; Cardoso, L.; Bourdeau, P.; Baneth, G.; Solano-Gallego, L.; Pennisi, M.G.; Ferrer, L.; Oliva, G. Novel Areas for Prevention and Control of Canine Leishmaniosis. Trends Parasitol. 2017, 33, 718–730. [Google Scholar] [CrossRef]
- Zutshi, S.; Kumar, S.; Chauhan, P.; Bansode, Y.; Nair, A.; Roy, S.; Sarkar, A.; Saha, B. Anti-Leishmanial Vaccines: Assumptions, Approaches, and Annulments. Vaccines (Basel) 2019, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against Leishmania under current research. Expert Rev. Vaccines 2018, 1–12. [Google Scholar] [CrossRef]
- Hohman, L.S.; Peters, N.C. CD4 (+) T Cell-Mediated Immunity against the Phagosomal Pathogen Leishmania: Implications for Vaccination. Trends Parasitol. 2019, 35, 423–435. [Google Scholar] [CrossRef]
- Mohebali, M.; Nadim, A.; Khamesipour, A. An overview of leishmanization experience: A successful control measure and a tool to evaluate candidate vaccines. Acta Trop. 2019, 200, 105173. [Google Scholar] [CrossRef] [PubMed]
- Mandell, M.A.; Beverley, S.M. Continual renewal and replication of persistent Leishmania major parasites in concomitantly immune hosts. Proc. Natl. Acad. Sci. USA 2017, 114, E801–E810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, D.L. Vaccines against tropical parasitic diseases: A persisting answer to a persisting problem. Nat. Immunol. 2014, 15, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Dunning, N. Leishmania vaccines: From leishmanization to the era of DNA technology. Biosci. Horiz. 2009, 2, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Chhajer, R.; Ali, N. Genetically modified organisms and visceral leishmaniasis. Front. Immunol. 2014, 5, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folgueira, C.; Quijada, L.; Soto, M.; Abanades, D.R.; Alonso, C.; Requena, J.M. The translational efficiencies of the two Leishmania infantum HSP70 mRNAs, differing in their 3’-Untranslated Regions, are affected by shifts in the temperature of growth through different mechanisms. J. Biol. Chem. 2005, 280, 35172–35183. [Google Scholar] [CrossRef] [Green Version]
- Folgueira, C.; Carrion, J.; Moreno, J.; Saugar, J.M.; Canavate, C.; Requena, J.M. Effects of the disruption of the HSP70-II gene on the growth, morphology, and virulence of Leishmania infantum promastigotes. Int. Microbiol. 2008, 11, 81–89. [Google Scholar] [CrossRef]
- Solana, J.C.; Ramirez, L.; Corvo, L.; de Oliveira, C.I.; Barral-Netto, M.; Requena, J.M.; Iborra, S.; Soto, M. Vaccination with a Leishmania infantum HSP70-II null mutant confers long-term protective immunity against Leishmania major infection in two mice models. PLoS Negl. Trop. Dis. 2017, 11, e0005644. [Google Scholar] [CrossRef] [Green Version]
- Calvo-Alvarez, E.; Guerrero, N.A.; Alvarez-Velilla, R.; Prada, C.F.; Requena, J.M.; Punzon, C.; Llamas, M.A.; Arevalo, F.J.; Rivas, L.; Fresno, M.; et al. Appraisal of a Leishmania major strain stably expressing mCherry fluorescent protein for both in vitro and in vivo studies of potential drugs and vaccine against cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2012, 6, e1927. [Google Scholar] [CrossRef] [Green Version]
- Carrion, J.; Folgueira, C.; Soto, M.; Fresno, M.; Requena, J.M. Leishmania infantum HSP70-II null mutant as candidate vaccine against leishmaniasis: A preliminary evaluation. Parasit. Vectors 2011, 4, 150. [Google Scholar] [CrossRef] [Green Version]
- Loeuillet, C.; Banuls, A.L.; Hide, M. Study of Leishmania pathogenesis in mice: Experimental considerations. Parasit. Vectors 2016, 9, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Velilla, R.; Gutierrez-Corbo, M.D.C.; Punzon, C.; Perez-Pertejo, M.Y.; Balana-Fouce, R.; Fresno, M.; Reguera, R.M. A chronic bioluminescent model of experimental visceral leishmaniasis for accelerating drug discovery. PLoS Negl. Trop. Dis. 2019, 13, e0007133. [Google Scholar] [CrossRef] [PubMed]
- Buffet, P.A.; Sulahian, A.; Garin, Y.J.; Nassar, N.; Derouin, F. Culture microtitration: A sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 1995, 39, 2167–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrion, J.; Nieto, A.; Iborra, S.; Iniesta, V.; Soto, M.; Folgueira, C.; Abanades, D.R.; Requena, J.M.; Alonso, C. Immunohistological features of visceral leishmaniasis in BALB/c mice. Parasite Immunol. 2006, 28, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J. Assessment of vaccine-induced immunity against canine visceral leishmaniasis. Front. Vet. Sci. 2019, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Filardy, A.A.; Guimaraes-Pinto, K.; Nunes, M.P.; Zukeram, K.; Fliess, L.; Pereira, L.; Oliveira Nascimento, D.; Conde, L.; Morrot, A. Human kinetoplastid protozoan infections: Where are we going next? Front. Immunol. 2018, 9, 1493. [Google Scholar] [CrossRef]
- Gillespie, P.M.; Beaumier, C.M.; Strych, U.; Hayward, T.; Hotez, P.J.; Bottazzi, M.E. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 2016, 34, 2992–2995. [Google Scholar] [CrossRef]
- Mendonca, S.C. Differences in immune responses against Leishmania induced by infection and by immunization with killed parasite antigen: Implications for vaccine discovery. Parasit. Vectors 2016, 9, 492. [Google Scholar] [CrossRef] [Green Version]
- Okwor, I.; Uzonna, J. Persistent parasites and immunologic memory in cutaneous leishmaniasis: Implications for vaccine designs and vaccination strategies. Immunol. Res. 2008, 41, 123–136. [Google Scholar] [CrossRef]
- Okwor, I.; Mou, Z.; Liu, D.; Uzonna, J. Protective immunity and vaccination against cutaneous leishmaniasis. Front. Immunol. 2012, 3, 128. [Google Scholar] [CrossRef] [Green Version]
- Beattie, L.; Evans, K.J.; Kaye, P.M.; Smith, D.F. Transgenic Leishmania and the immune response to infection. Parasite Immunol. 2008, 30, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saljoughian, N.; Taheri, T.; Rafati, S. Live vaccination tactics: Possible approaches for controlling visceral leishmaniasis. Front Immunol. 2014, 5, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkaid, Y.; Mendez, S.; Lira, R.; Kadambi, N.; Milon, G.; Sacks, D. A natural model of Leishmania major infection reveals a prolonged "silent" phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 2000, 165, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handman, E. Leishmaniasis: Current status of vaccine development. Clin. Microbiol. Rev. 2001, 14, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Nylen, S. Immunobiology of visceral leishmaniasis. Front. Immunol. 2012, 3, 251. [Google Scholar] [CrossRef] [Green Version]
- Kaye, P.M.; Beattie, L. Lessons from other diseases: Granulomatous inflammation in leishmaniasis. Semin. Immunopathol. 2016, 38, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Nieto, A.; Dominguez-Bernal, G.; Orden, J.A.; De La Fuente, R.; Madrid-Elena, N.; Carrion, J. Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus syrian hamster model. Vet. Res. 2011, 42, 39. [Google Scholar] [CrossRef] [Green Version]
- Beattie, L.; Kaye, P.M. Leishmania-host interactions: What has imaging taught us? Cell Microbiol. 2011, 13, 1659–1667. [Google Scholar] [CrossRef]
- Salguero, F.J.; Garcia-Jimenez, W.L.; Lima, I.; Seifert, K. Histopathological and immunohistochemical characterisation of hepatic granulomas in Leishmania donovani-infected BALB/c mice: A time-course study. Parasit. Vectors 2018, 11, 73. [Google Scholar] [CrossRef]
- Kedzierski, L.; Evans, K.J. Immune responses during cutaneous and visceral leishmaniasis. Parasitology 2014, 1–19. [Google Scholar] [CrossRef]
- Squires, K.E.; Schreiber, R.D.; McElrath, M.J.; Rubin, B.Y.; Anderson, S.L.; Murray, H.W. Experimental visceral leishmaniasis: Role of endogenous IFN-gamma in host defense and tissue granulomatous response. J. Immunol. 1989, 143, 4244–4249. [Google Scholar] [PubMed]
- Selvapandiyan, A.; Dey, R.; Gannavaram, S.; Lakhal-Naouar, I.; Duncan, R.; Salotra, P.; Nakhasi, H.L. Immunity to visceral leishmaniasis using genetically defined live-attenuated parasites. J. Trop. Med. 2012, 2012, 631460. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.; Cordeiro-da-Silva, A.; Laforge, M.; Silvestre, R.; Estaquier, J. Regulation of immunity during visceral Leishmania infection. Parasit. Vectors 2016, 9, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stager, S.; Rafati, S. CD8 (+) T cells in leishmania infections: Friends or foes? Front. Immunol. 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, K.; Jain, N.K. Vaccines for visceral leishmaniasis: A review. J. Immunol. Methods. 2015, 422, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Doria, N.A.; Mendez, J.; Sacks, D.L.; Peters, N.C. Cutaneous infection with Leishmania major mediates heterologous protection against visceral infection with Leishmania infantum. J. Immunol. 2015, 195, 3816–3827. [Google Scholar] [CrossRef] [Green Version]
- Selvapandiyan, A.; Duncan, R.; Debrabant, A.; Bertholet, S.; Sreenivas, G.; Negi, N.S.; Salotra, P.; Nakhasi, H.L. Expression of a mutant form of Leishmania donovani centrin reduces the growth of the parasite. J. Biol. Chem. 2001, 276, 43253–43261. [Google Scholar] [CrossRef] [Green Version]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef] [Green Version]
- Rolao, N.; Cortes, S.; Gomes-Pereira, S.; Campino, L. Leishmania infantum: Mixed T-helper-1/T-helper-2 immune response in experimentally infected BALB/c mice. Exp. Parasitol. 2007, 115, 270–276. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Dey, R.; Nylen, S.; Duncan, R.; Sacks, D.; Nakhasi, H.L. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J. Immunol. 2009, 183, 1813–1820. [Google Scholar] [CrossRef] [Green Version]
- Bunn, P.T.; Montes de Oca, M.; de Labastida Rivera, F.; Kumar, R.; Ng, S.S.; Edwards, C.L.; Faleiro, R.J.; Sheel, M.; Amante, F.H.; Frame, T.C.M.; et al. Distinct Roles for CD4 (+) Foxp3 (+) Regulatory T Cells and IL-10-Mediated Immunoregulatory mechanisms during experimental visceral leishmaniasis caused by Leishmania donovani. J. Immunol. 2018, 201, 3362–3372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stager, S.; Joshi, T.; Bankoti, R. Immune evasive mechanisms contributing to persistent Leishmania donovani infection. Immunol. Res. 2010, 47, 14–24. [Google Scholar] [CrossRef]
- Nylen, S.; Sacks, D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007, 28, 378–384. [Google Scholar] [CrossRef]
- Silvestre, R.; Cordeiro-Da-Silva, A.; Santarem, N.; Vergnes, B.; Sereno, D.; Ouaissi, A. SIR2-Deficient Leishmania infantum induces a defined IFN-gamma/IL-10 Pattern that correlates with protection. J. Immunol. 2007, 179, 3161–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarlane, E.; Mokgethi, T.; Kaye, P.M.; Hurdayal, R.; Brombacher, F.; Alexander, J.; Carter, K.C. IL-4 mediated resistance of BALB/c mice to visceral leishmaniasis is independent of IL-4Ralpha signaling via T Cells. Front. Immunol. 2019, 10, 1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodhale, N.P.; Pal, S.; Kumar, S.; Chattopadhyay, D.; Saha, B.; Chattopadhyay, N.; Bhattacharyya, M. Inbred mouse strains differentially susceptible to Leishmania donovani infection differ in their immune cell metabolism. Cytokine 2018, 112, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Madhubala, R. Genetically engineered ascorbic acid-deficient live mutants of Leishmania donovani induce long lasting protective immunity against visceral leishmaniasis. Sci. Rep. 2015, 5, 10706. [Google Scholar] [CrossRef]
- Bhowmick, S.; Ravindran, R.; Ali, N. IL-4 contributes to failure, and colludes with IL-10 to exacerbate Leishmania donovani infection following administration of a subcutaneous leishmanial antigen vaccine. BMC Microbiol. 2014, 14, 8. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Kaur, T.; Garg, N.; Mukherjee, S.; Raina, P.; Athokpam, V. Effect of dose and route of inoculation on the generation of CD4+ Th1/Th2 type of immune response in murine visceral leishmaniasis. Parasitol. Res. 2008, 103, 1413–1419. [Google Scholar] [CrossRef]
- Fernandez Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Maranon, F.; Fabra, M.; Gomez-Nieto, L.C.; Alonso, C. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend(R) against canine leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Carcelen, J.; Iniesta, V.; Fernandez-Cotrina, J.; Serrano, F.; Parejo, J.C.; Corraliza, I.; Gallardo-Soler, A.; Maranon, F.; Soto, M.; Alonso, C.; et al. The chimerical multi-component Q protein from Leishmania in the absence of adjuvant protects dogs against an experimental Leishmania infantum infection. Vaccine 2009, 27, 5964–5973. [Google Scholar] [CrossRef] [PubMed]
- Poot, J.; Janssen, L.H.; van Kasteren-Westerneng, T.J.; van der Heijden-Liefkens, K.H.; Schijns, V.E.; Heckeroth, A. Vaccination of dogs with six different candidate leishmaniasis vaccines composed of a chimerical recombinant protein containing ribosomal and histone protein epitopes in combination with different adjuvants. Vaccine 2009, 27, 4439–4446. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solana, J.C.; Ramírez, L.; Cook, E.C.; Hernández-García, E.; Sacristán, S.; Martín, M.E.; Manuel González, V.; Reguera, R.M.; Balaña-Fouce, R.; Fresno, M.; et al. Subcutaneous Immunization of Leishmania HSP70-II Null Mutant Line Reduces the Severity of the Experimental Visceral Leishmaniasis in BALB/c Mice. Vaccines 2020, 8, 141. https://doi.org/10.3390/vaccines8010141
Solana JC, Ramírez L, Cook EC, Hernández-García E, Sacristán S, Martín ME, Manuel González V, Reguera RM, Balaña-Fouce R, Fresno M, et al. Subcutaneous Immunization of Leishmania HSP70-II Null Mutant Line Reduces the Severity of the Experimental Visceral Leishmaniasis in BALB/c Mice. Vaccines. 2020; 8(1):141. https://doi.org/10.3390/vaccines8010141
Chicago/Turabian StyleSolana, José Carlos, Laura Ramírez, Emma CL Cook, Elena Hernández-García, Silvia Sacristán, M. Elena Martín, Víctor Manuel González, Rosa María Reguera, Rafael Balaña-Fouce, Manuel Fresno, and et al. 2020. "Subcutaneous Immunization of Leishmania HSP70-II Null Mutant Line Reduces the Severity of the Experimental Visceral Leishmaniasis in BALB/c Mice" Vaccines 8, no. 1: 141. https://doi.org/10.3390/vaccines8010141
APA StyleSolana, J. C., Ramírez, L., Cook, E. C., Hernández-García, E., Sacristán, S., Martín, M. E., Manuel González, V., Reguera, R. M., Balaña-Fouce, R., Fresno, M., Requena, J. M., Iborra, S., & Soto, M. (2020). Subcutaneous Immunization of Leishmania HSP70-II Null Mutant Line Reduces the Severity of the Experimental Visceral Leishmaniasis in BALB/c Mice. Vaccines, 8(1), 141. https://doi.org/10.3390/vaccines8010141