Alpha-Gal and Cross-Reactive Carbohydrate Determinants in the N-Glycans of Salivary Glands in the Lone Star Tick, Amblyomma americanum
Abstract
:1. Introduction
2. Materials and Methods
2.1. N-Glycan Profiling
2.2. Release of N-Linked Glycans
2.3. Per-O-Methylation of N-Linked Glycans
2.4. Profiling and Fragmentation Analysis by Nanospray Ionization-Mass Spectrometry (NSI-FTMS/MS)
2.5. Bioinformatics
3. Results
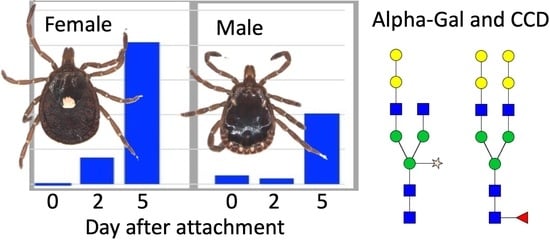
3.1. Spatial and Temporal Changes in the N-Glycosylation Patterns in the Salivary Glands of Male and Female A. americanum
3.2. Genes Encoding the Enzymes for N-Glycan Processing
4. Discussion
4.1. Dynamic Control of the N-Glycosylation Patterns in Tick SGs
4.2. Cross-Reactive Carbohydrate Determinants (CCDs) and aGal in Tick N-glycans
4.3. aGal in the Tick SG as the Sensitizer of Red Meat Allergy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helenius, A.; Aebi, M. Intracellular functions of N-linked glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenborre, G.; Smagghe, G.; Ghesquiere, B.; Menschaert, G.; Nagender Rao, R.; Gevaert, K.; Van Damme, E.J. Diversity in protein glycosylation among insect species. PLoS ONE 2011, 6, e16682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheys, F.; De Schutter, K.; Shen, Y.; Yu, N.; Smargiasso, N.; De Pauw, E.; Van Damme, E.J.M.; Smagghe, G. The N-glycome of the hemipteran pest insect Nilaparvata lugens reveals unexpected sex differences. Insect Biochem. Mol. Biol. 2019, 107, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Walski, T.; Van Damme, E.J.M.; Smargiasso, N.; Christiaens, O.; De Pauw, E.; Smagghe, G. Protein N-glycosylation and N-glycan trimming are required for postembryonic development of the pest beetle Tribolium castaneum. Sci. Rep. 2016, 6, 35151. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; De Schutter, K.; Van Damme, E.J.M.; Smagghe, G. Synthesis and biological roles of O-glycans in insects. Glycoconj. J. 2019. [Google Scholar] [CrossRef]
- De Pourcq, K.; De Schutter, K.; Callewaert, N. Engineering of glycosylation in yeast and other fungi: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 87, 1617–1631. [Google Scholar] [CrossRef]
- Gagneux, P.; Aebi, M.; Varki, A. Evolution of glycan diversity. In Essentials of Glycobiology, 3rd ed.; Varki, A.C.R., Esko, J.D., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- De Schutter, K.; Van Damme, E.J.M. Protein-carbohydrate interactions as part of plant defense and animal immunity. Molecules 2015, 20, 9029–9053. [Google Scholar] [CrossRef] [Green Version]
- Delaloye, J.; Calandra, T. Host innate immune responses to microbial pathogens. Curr. Vasc. Pharmacol. 2013, 11, 123–132. [Google Scholar]
- Cummings, R.D.; McEver, R.P. C-Type Lectins. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Kinoshita, A.G.D.T., Packer, N.H., Prestegard, J.H., Eds.; Cold Spring Harbor: New York, NY, USA, 2017. [Google Scholar]
- Khatua, B.; Ghoshal, A.; Bhattacharya, K.; Mandal, C.; Saha, B.; Crocker, P.R.; Mandal, C. Sialic acids acquired by Pseudomonas aeruginosa are involved in reduced complement deposition and siglec mediated host-cell recognition. FEBS Lett. 2010, 584, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Carlin, A.F.; Uchiyama, S.; Chang, Y.C.; Lewis, A.L.; Nizet, V.; Varki, A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 2009, 113, 3333–3336. [Google Scholar] [CrossRef]
- Freire-De-Lima, L.; Oliveira, I.A.; Neves, J.L.; Penha, L.L.; Alisson-Silva, F.; Dias, W.B.; Todeschini, A.R. Sialic acid: A sweet swing between mammalian host and Trypanosoma cruzi. Front. Immunol. 2012, 3, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Previato, J.O.; Andrade, A.F.B.; Pessolani, M.C.V.; Mendoncapreviato, L. Incorporation of sialic-acid into Trypanosoma cruzi macromolecules—A proposal for a new metabolic route. Mol. Biochem. Parasit. 1985, 16, 85–96. [Google Scholar] [CrossRef]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immun. 2012, 42, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Pena, A.; Horak, I.G.; Shao, R.F.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Simo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef]
- Chen, Z.C.; Radic, M.Z.; Galili, U. Genes coding evolutionary novel anti-carbohydrate antibodies: Studies on anti-Gal production in alpha 1,3galactosyltransferase knock out mice. Mol. Immunol. 2000, 37, 455–466. [Google Scholar] [CrossRef]
- Commins, S.P.; Jerath, M.R.; Cox, K.; Erickson, L.D.; Platts-Mills, T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol. Int. 2016, 65, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Galili, U. Evolution of alpha 1, 3 galactosyltransferase and of the alpha-Gal epitope. Subcell. Biochem. 1999, 32, 1–23. [Google Scholar]
- Commins, S.P.; Platts-Mills, T.A. Tick bites and red meat allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Hamsten, C.; Tran, T.A.; Starkhammar, M.; Brauner, A.; Commins, S.P.; Platts-Mills, T.A.; van Hage, M. Red meat allergy in Sweden: Association with tick sensitization and B-negative blood groups. J. Allergy. Clin. Immunol. 2013, 132, 1431–1434. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Simo, L.; Park, Y. Molecular characterization of neuropeptide elevenin and two elevenin receptors, IsElevRl and IsElevR2, from the blacklegged tick, Ixodes scapularis. Insect Biochem. Mol. Biol. 2018, 101, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Krober, T.; Guerin, P.M. In vitro feeding assays for hard ticks. Trends Parasitol. 2007, 23, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shajahan, A.; Heiss, C.; Ishihara, M.; Azadi, P. Glycomic and glycoproteomic analysis of glycoproteins-a tutorial. Anal. Bioanal. Chem. 2017, 409, 4483–4505. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method—A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence-limits on phylogenies—An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Gladney, W.J.; Drummond, R.O. Migration of male lone star ticks on host in relation to mating. J. Econ. Entomol. 1970, 63, 1214. [Google Scholar] [CrossRef]
- van Ree, R.; Cabanes-Macheteau, M.; Akkerdaas, J.; Milazzo, J.P.; Loutelier-Bourhis, C.; Rayon, C.; Villalba, M.; Koppelman, S.; Aalberse, R.; Rodriguez, R.; et al. Beta (1, 2)-xylose and alpha (1, 3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J. Biol. Chem. 2000, 275, 11451–11458. [Google Scholar] [CrossRef] [Green Version]
- Altmann, F. Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergy J. Int. 2016, 25, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Dragosits, M.; Yan, S.; Razzazi-Fazeli, E.; Wilson, I.B.H.; Rendic, D. Enzymatic properties and subtle differences in the substrate specificity of phylogenetically distinct invertebrate N-glycan processing hexosaminidases. Glycobiology 2015, 25, 448–464. [Google Scholar] [CrossRef]
- Zhu, F.F.; Li, D.; Chen, K.P. Structures and functions of invertebrate glycosylation. Open Biol. 2019, 9, 180232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabezas-Cruz, A.; Espinosa, P.J.; Alberdi, P.; Simo, L.; Valdes, J.J.; Mateos-Hernandez, L.; Contreras, M.; Rayo, M.V.; de la Fuente, J. Tick galactosyltransferases are involved in alpha-Gal synthesis and play a role during Anaplasma phagocytophilum infection and Ixodes scapularis tick vector development. Sci. Rep. 2018, 8, 14224. [Google Scholar] [CrossRef] [PubMed]
- Rendic, D.; Wilson, I.B.H.; Paschinger, K. The glycosylation capacity of insect cells. Croat. Chem. Acta 2008, 81, 7–21. [Google Scholar]
- Kolarich, D.; Leonard, R.; Hemmer, W.; Altmann, F. The N-glycans of yellow jacket venom hyaluronidases and the protein sequence of its major isoform in Vespula vulgaris. FEBS J. 2005, 272, 5182–5190. [Google Scholar] [CrossRef]
- Aoki, K.; Tiemeyer, M. The glycomics of glycan glucuronylation in Drosophila melanogaster. Method Enzymol. 2010, 480, 297–321. [Google Scholar] [CrossRef]
- Hagen, K.G.T.; Zhang, L.P.; Tian, E.; Zhang, Y. Glycobiology on the fly: Developmental and mechanistic insights from Drosophila. Glycobiology 2009, 19, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Katoh, T.; Tiemeyer, M. The N’s and O’s of Drosophila glycoprotein glycobiology. Glycoconj. J. 2013, 30, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Kurz, S.; Aoki, K.; Jin, C.; Karlsson, N.G.; Tiemeyer, M.; Wilson, I.B.; Paschinger, K. Targeted release and fractionation reveal glucuronylated and sulphated N-and O-glycans in larvae of dipteran insects. J. Proteom. 2015, 126, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Tirloni, L.; Pinto, A.F.M.; Moresco, J.; Yates, J.R.; Vaz, I.D.S.; Mulenga, A. Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS. Negl. Trop. Dis. 2016, 10, e0004323. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.J.; Anatriello, E.; de Miranda-Santos, I.K.; Francischetti, I.M.; Sa-Nunes, A.; Ferreira, B.R.; Ribeiro, J.M.C. Proteome of Rhipicephalus sanguineus tick saliva induced by the secretagogues pilocarpine and dopamine. Ticks Tick-Borne Dis. 2013, 4, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, H.; Galili, U. Profiling terminal N-acetyllactosamines of glycans on mammalian cells by an immuno-enzymatic assay. Glycoconj. J. 2006, 23, 663–674. [Google Scholar] [CrossRef]
- Tan, Y.X.; Gong, F.; Li, S.B.; Ji, S.P.; Lu, Y.P.; Gao, H.W.; Xu, H.; Zhang, Y.P. Brief report: A new profile of terminal N-acetyllactosamines glycans on pig red blood cells and different expression of alpha-galactose on Sika deer red blood cells and nucleated cells. Glycoconj. J. 2010, 27, 427–433. [Google Scholar] [CrossRef]
- Homann, A.; Schramm, G.; Jappe, U. Glycans and glycan-specific IgE in clinical and molecular allergology: Sensitization, diagnostics, and clinical symptoms. J. Allergy Clin. Immunol. 2017, 140, 356–368. [Google Scholar] [CrossRef] [Green Version]
- van Nunen, S. Tick-induced allergies: Mammalian meat allergy, tick anaphylaxis and their significance. Asia Pac. Allergy 2015, 5, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Jerath, M.R.; Commins, S.P. Venom allergy is increased in alpha-gal allergy: Shared environmental or immunologic factors? J. Allergy Clin. Immun. 2018, 141, AB199. [Google Scholar] [CrossRef]
- Yokoi, H.; Yoshitake, H.; Matsumoto, Y.; Kawada, M.; Takato, Y.; Shinagawa, K.; Sakurai, H.; Saito, K. Involvement of cross-reactive carbohydrate determinants-specific IgE in pollen allergy testing. Asia Pac. Allergy 2017, 7, 29–36. [Google Scholar] [CrossRef]
- Hemmer, W.; Altmann, F.; Holzweber, F.; Gruber, C.; Wantke, F.; Wohrl, S. ImmunoCAP cellulose displays cross-reactive carbohydrate determinant (CCD) epitopes and can cause false-positive test results in patients with high anti-CCD IgE antibody levels. J. Allergy Clin. Immun. 2018, 141, 372. [Google Scholar] [CrossRef] [Green Version]
- Van Nunen, S.A.; O’Connor, K.S.; Clarke, L.R.; Boyle, R.X.; Fernando, S.L. An association between tick bite reactions and red meat allergy in humans. Med. J. Aust. 2009, 190, 510–511. [Google Scholar] [CrossRef]
- Jappe, U.; Minge, S.; Kreft, B.; Ludwig, A.; Przybilla, B.; Walker, A.; Varga, R.; Seidel, P.; Biedermann, T.; Anemuller, W.; et al. Meat allergy associated with galactosyl-(1,3)-galactose (-Gal) Closing diagnostic gaps by anti-Gal IgE immune profiling. Allergy 2018, 73, 93–105. [Google Scholar] [CrossRef]
- Araujo, R.N.; Franco, P.F.; Rodrigues, H.; Santos, L.C.; McKay, C.S.; Sanhueza, C.A.; Brito, C.R.; Azevedo, M.A.; Venuto, A.P.; Cowan, P.J.; et al. Amblyomma sculptum tick saliva: Alpha-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int. J. Parasitol. 2016, 46, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Crispell, G.; Commins, S.P.; Archer-Hartman, S.A.; Choudhary, S.; Dharmarajan, G.; Azadi, P.; Karim, S. Discovery of alpha-Gal-containing antigens in north American tick species believed to induce red meat allergy. Front. Immunol. 2019, 10, 1056. [Google Scholar] [CrossRef] [Green Version]
- Cabezas-Cruz, A.; Hodzic, A.; Roman-Carrasco, P.; Mateos-Hernandez, L.; Duscher, G.G.; Sinha, D.K.; Hemmer, W.; Swoboda, I.; Estrada-Pena, A.; de la Fuente, J. Environmental and molecular drivers of the alpha-Gal syndrome. Front. Immunol. 2019, 10, 1210. [Google Scholar] [CrossRef] [Green Version]
- Hilger, C.; Fischer, J.; Wolbing, F.; Biedermann, T. Role and mechanism of galactose-alpha-1, 3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Curr. Allergy Asthma Rep. 2019, 19, 3. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Fulton, R.B.; O’Connell, R.; Jang, H.S.; Fernando, S.L. Specific-IgE to galactose-alpha-1, 3-galactose (alpha-gal) has limited utility in diagnosing meat allergy in a tick-endemic population. Ann. Allergy Asthma Immunol. 2018, 121, 509–511. [Google Scholar] [CrossRef]
- Wilson, J.M.; Schuyler, A.J.; Workman, L.; Gupta, M.; James, H.R.; Posthumus, J.; McGowan, E.C.; Commins, S.P.; Platts-Mills, T.A.E. Investigation into the alpha-Gal syndrome: Characteristics of 261 children and adults reporting red meat allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 2348–2358. [Google Scholar] [CrossRef]
- Dahl, K.; Buschard, K.; Gram, D.X.; d’Apice, A.J.; Hansen, A.K. Glucose intolerance in a xenotransplantation model: Studies in alpha-gal knockout mice. APMIS 2006, 114, 805–811. [Google Scholar] [CrossRef]
- Ayoub, D.; Jabs, W.; Resemann, A.; Evers, W.; Evans, C.; Main, L.; Baessmann, C.; Wagner-Rousset, E.; Suckau, D.; Beck, A. Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. mAbs 2013, 5, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Galili, U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol. Cell Biol. 2005, 83, 674–686. [Google Scholar] [CrossRef]
- Roman-Carrasco, P.; Lieder, B.; Somoza, V.; Ponce, M.; Szepfalusi, Z.; Martin, D.; Hemmer, W.; Swoboda, I. Only alpha-Gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE-reactive molecule that can induce effector cell activation. Allergy 2019, 10, 1956–1968. [Google Scholar] [CrossRef]
- Commins, S.P.; James, H.R.; Kelly, L.A.; Pochan, S.L.; Workman, L.J.; Perzanowski, M.S.; Kocan, K.M.; Fahy, J.V.; Nganga, L.W.; Ronmark, E.; et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1, 3-galactose. J. Allergy Clin. Immunol. 2011, 127, 1286–1293.e6. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Nuttall, P.A. Excretion of host immunoglobulin in tick saliva and detection of IgG-binding proteins in tick haemolymph and salivary glands. Parasitology 1994, 109, 525–530. [Google Scholar] [CrossRef]
- Wang, H.; Nuttall, P.A. Immunoglobulin-binding proteins in ticks: New target for vaccine development against a blood-feeding parasite. Cell Mol. Life Sci. 1999, 56, 286–295. [Google Scholar] [CrossRef]
- Friesen, K.J.; Kaufman, W.R. Salivary gland degeneration and vitellogenesis in the ixodid tick Amblyomma hebraeum: Surpassing a critical weight is the prerequisite and detachment from the host is the trigger. J. Insect Physiol. 2009, 55, 936–942. [Google Scholar] [CrossRef]
- Avila, J.L.; Rojas, M.; Galili, U. Immunogenic Gal-alpha-1-3gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J. Immunol. 1989, 142, 2828–2834. [Google Scholar]
- Zamze, S.E.; Ashford, D.A.; Wooten, E.W.; Rademacher, T.W.; Dwek, R.A. Structural characterization of the asparagine-linked oligosaccharides from Trypanosoma brucei Type-Ii and Type-Iii variant surface glycoproteins. J. Biol. Chem. 1991, 266, 20244–20261. [Google Scholar]
- Cabezas-Cruz, A.; de la Fuente, J. Immunity to alpha-Gal: Toward a single-antigen pan-vaccine to control major infectious diseases. ACS Cent. Sci. 2017, 3, 1140–1142. [Google Scholar] [CrossRef]
- Lei, Y.; Yu, H.; Dong, Y.; Yang, J.; Ye, W.; Wang, Y.; Chen, W.; Jia, Z.; Xu, Z.; Li, Z.; et al. Characterization of N-glycan structures on the surface of mature Dengue 2 virus derived from insect cells. PLoS ONE 2015, 10, e0132122. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.; Portugal, S.; Tran, T.M.; Gozzelino, R.; Ramos, S.; Gomes, J.; Regalado, A.; Cowan, P.J.; d’Apice, A.J.F.; Chong, A.S.; et al. Gut microbiota elicits a protective immune response against Malaria transmission. Cell 2014, 159, 1277–1289. [Google Scholar] [CrossRef] [Green Version]
- Moura, A.P.V.; Santos, L.C.B.; Brito, C.R.N.; Valencia, E.; Junqueira, C.; Filho, A.A.P.; Sant’Anna, M.R.V.; Gontijo, N.F.; Bartholomeu, D.C.; Fujiwara, R.T.; et al. Virus-like particle display of the alpha-Gal carbohydrate for vaccination against Leishmania infection. ACS Cent. Sci. 2017, 3, 1026–1031. [Google Scholar] [CrossRef] [Green Version]
- Almeida, I.C.; Milani, S.R.; Gorin, P.A.J.; Travassos, L.R. Complement-mediated lysis of Trypanosoma. cruzi. trypomastigotes by human anti-alpha-galactosyl antibodies. J. Immunol. 1991, 146, 2394–2400. [Google Scholar]
- Cabezas-Cruz, A.; de la Fuente, J. Immunity to alpha-Gal: The opportunity for malaria and Tuberculosis control. Front. Immunol. 2017, 8, 1733. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Kim, D.; Boorgula, G.D.; De Schutter, K.; Smagghe, G.; Šimo, L.; Archer-Hartmann, S.A.; Azadi, P. Alpha-Gal and Cross-Reactive Carbohydrate Determinants in the N-Glycans of Salivary Glands in the Lone Star Tick, Amblyomma americanum. Vaccines 2020, 8, 18. https://doi.org/10.3390/vaccines8010018
Park Y, Kim D, Boorgula GD, De Schutter K, Smagghe G, Šimo L, Archer-Hartmann SA, Azadi P. Alpha-Gal and Cross-Reactive Carbohydrate Determinants in the N-Glycans of Salivary Glands in the Lone Star Tick, Amblyomma americanum. Vaccines. 2020; 8(1):18. https://doi.org/10.3390/vaccines8010018
Chicago/Turabian StylePark, Yoonseong, Donghun Kim, Gunavanthi D. Boorgula, Kristof De Schutter, Guy Smagghe, Ladislav Šimo, Stephanie A. Archer-Hartmann, and Parastoo Azadi. 2020. "Alpha-Gal and Cross-Reactive Carbohydrate Determinants in the N-Glycans of Salivary Glands in the Lone Star Tick, Amblyomma americanum" Vaccines 8, no. 1: 18. https://doi.org/10.3390/vaccines8010018
APA StylePark, Y., Kim, D., Boorgula, G. D., De Schutter, K., Smagghe, G., Šimo, L., Archer-Hartmann, S. A., & Azadi, P. (2020). Alpha-Gal and Cross-Reactive Carbohydrate Determinants in the N-Glycans of Salivary Glands in the Lone Star Tick, Amblyomma americanum. Vaccines, 8(1), 18. https://doi.org/10.3390/vaccines8010018