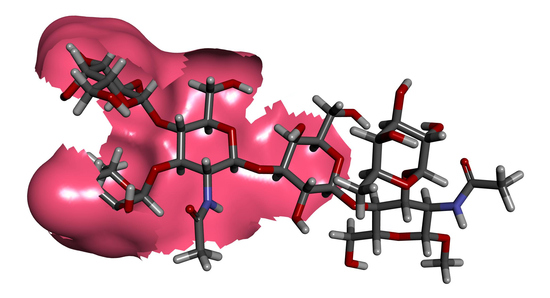
Recognition of Dimeric Lewis X by Anti-Dimeric Lex Antibody SH2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ascites Containing mAb SH2
2.2. Preparation of the GDimLex-BSA (5) Glycoconjugate
2.3. Indirect Titration ELISA Procedures
2.4. Competitive ELISA Procedures
3. Results
3.1. Titration Experiments
3.2. Competitive Inhibition Studies with DimLex Fragments and Glycoconjugates
3.3. Competitive Inhibition Binding Studies with Lex Analogues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soliman, C.; Yuriev, E.; Ramsland, P.A. Antibody recognition of aberrant glycosylation on the surface of cancer cells. Curr. Opin. Struct. Biol. 2007, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S.-I. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, A.K.; Ørntoft, T.F.; Nudelman, E.; Nance, S.; Schibig, L.; Stroud, M.R.; Clausen, H.; Hakomori, S. Profiles of Lewisx-containing Glycoproteins and Glycolipids in Sera of Patients with Adenocarcinoma. Cancer Res. 1990, 50, 1375–1380. [Google Scholar] [PubMed]
- Hakomori, S.; Kannagi, R. Glycosphingolipids as Tumor-Associated and Differentiation Markers. J. Natl. Cancer Inst. 1983, 71, 231–251. [Google Scholar]
- Hakomori, S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996, 56, 5309–5318. [Google Scholar]
- Yanagisawa, M.; Yu, R.K. The expression and functions of glycoconjugates in neural stem cells. Glycobiology 2007, 17, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Hakomori, S.; Jeanloz, R.W. Isolation of a Glycolipid Containing Fucose, Galactose, Glucose, and Glucosamine from Human Cancerous Tissue. J. Biol. Chem. 1964, 239, 3606–3607. [Google Scholar]
- Hakomori, S.; Nudelman, E.; Levery, S.B.; Kannagi, R. Novel fucolipids accumulating in human adenocarcinoma. I. Glycolipids with di- or trifucosylated type 2 chain. J. Biol. Chem. 1984, 259, 4672–4680. [Google Scholar]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent Advance in Tumor-associated Carbohydrate Antigens (TACAs)-based Antitumor Vaccines. ACS Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef]
- Hakomori, S.-I. Tumor-associated glycolipid antigens, their metabolism and organization. Chem. Phys. Lipids 1986, 42, 209–233. [Google Scholar] [CrossRef]
- Hakomori, S. Tumor-Associated Carbohydrate Antigens. Annu Rev. Immunol. 1984, 2, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Hakomori, S.-I. Molecular changes in carbohydrate antigens associated with cancer. Bioessays 1990, 12, 223–230. [Google Scholar] [CrossRef]
- Brockhaus, M.; Magnani, J.L.; Herlyn, M.; Blaszczyk, M.; Steplewski, Z.; Koprowski, H.; Ginsburg, V. Monoclonal antibodies directed against the sugar sequence of lacto-N-fucopentaose III are obtained from mice immunized with human tumors. Arch. Biochem. Biophys. 1982, 217, 647–651. [Google Scholar] [CrossRef]
- Cuttitta, F.; Rosen, S.; Gazdar, A.F.; Minna, J.D. Monoclonal-Antibodies That Demonstrate Specificity for Several Types of Human-Lung Cancer. Proc. Natl Acad Sci. Biol. USA 1981, 78, 4591–4595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.C.; Brockhaus, M.; Magnani, J.L.; Cuttitta, F.; Rosen, S.; Minna, J.D.; Ginsburg, V. Many monoclonal antibodies with an apparent specificity for certain lung cancers are directed against a sugar sequence found in lacto-N-fucopentaose III. Arch. Biochem. Biophys. 1983, 220, 318–320. [Google Scholar] [CrossRef]
- Huang, L.C.; Civin, C.I.; Magnani, J.L.; Shaper, J.H.; Ginsburg, V. My-1, the Human Myeloid-Specific Antigen Detected by Mouse Monoclonal-Antibodies, Is a Sugar Sequence Found in Lacto-N-Fucopentaose-Iii. Blood 1983, 61, 1020–1023. [Google Scholar] [CrossRef] [Green Version]
- Gooi, H.C.; Thorpe, S.J.; Hounsell, E.F.; Rumpold, H.; Kraft, D.; Forster, O.; Feizi, T. Marker of Peripheral-Blood Granulocytes and Monocytes of Man Recognized by 2 Monoclonal-Antibodies Vep8 and Vep9 Involves the Trisaccharide 3-Fucosyl-N-Acetyllactosamine. Eur. J. Immunol. 1983, 13, 306–312. [Google Scholar] [CrossRef]
- Urdal, D.L.; Brentnall, T.A.; Bernstein, I.D.; Hakomori, S.I. A granulocyte reactive monoclonal antibody, 1G10, identifies the Gal beta 1-4 (Fuc alpha 1-3) GlcNAc (X determinant) expressed in HL-60 cells on both glycolipid and glycoprotein molecules. Blood 1983, 62, 1022–1026. [Google Scholar] [CrossRef] [Green Version]
- Combs, S.G.; Marder, R.J.; Minna, J.D.; Mulshine, J.L.; Polovina, M.R.; Rosen, S.T. Immunohistochemical Localization of the Immunodominant Differentiation Antigen Lacto-N-Fucopentaose-Iii in Normal Adult and Fetal Tissues. J. Histochem. Cytochem. 1984, 32, 982–988. [Google Scholar] [CrossRef] [Green Version]
- Solter, D.; Knowles, B.B. Monoclonal Antibody Defining a Stage-Specific Mouse Embryonic Antigen (Ssea-1). Proc. Natl. Acad. Sci. USA 1978, 75, 5565–5569. [Google Scholar] [CrossRef] [Green Version]
- Hakomori, S.; Nudelman, E.; Levery, S.; Solter, D.; Knowles, B.B. The Hapten Structure of a Developmentally Regulated Glycolipid Antigen (Ssea-1) Isolated from Human-Erythrocytes and Adenocarcinoma—A Preliminary Note. Biochem. Biophys. Res. Commun. 1981, 100, 1578–1586. [Google Scholar] [CrossRef]
- Gooi, H.C.; Feizi, T.; Kapadia, A.; Knowles, B.B.; Solter, D.; Evans, M.J. Stage-Specific Embryonic Antigen Involves Alpha-1-]3 Fucosylated Type-2 Blood-Group Chains. Nature 1981, 292, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Kannagi, R.; Nudelman, E.; Levery, S.B.; Hakomori, S. A series of human erythrocyte glycosphingolipids reacting to the monoclonal antibody directed to a developmentally regulated antigen SSEA-1. J. Biol. Chem. 1982, 257, 14865–14874. [Google Scholar]
- Hakomori, S.; Nudelman, E.; Kannagi, R.; Levery, S.B. The Common Structure in Fucosyllactosaminolipids Accumulating in Human Adenocarcinomas, and Its Possible Absence in Normal Tissue. Biochem. Biophys. Res. Commun. 1982, 109, 36–44. [Google Scholar] [CrossRef]
- Fukushi, Y.; Hakomori, S.; Nudelman, E.; Cochran, N. Novel fucolipids accumulating in human adenocarcinoma. II. Selective isolation of hybridoma antibodies that differentially recognize mono-, di-, and trifucosylated type 2 chain. J. Biol. Chem. 1984, 259, 4681–4685. [Google Scholar]
- Fox, N.; Damjanov, I.; Knowles, B.B.; Solter, D. Immunohistochemical Localization of the Mouse Stage-specific Embryonic Antigen 1 in Human Tissues and Tumors. Cancer Res. 1983, 43, 669–678. [Google Scholar]
- Fukushi, Y.; Hakomori, S.; Shepard, T. Localization and alteration of mono-, di-, and trifucosyl alpha 1----3 type 2 chain structures during human embryogenesis and in human cancer. J. Exp. Med. 1984, 160, 506–520. [Google Scholar] [CrossRef] [Green Version]
- Van Roon, A.-M.M.; Van de Vijver, K.K.; Jacobs, W.; Van Marck, E.A.; Van Dam, G.J.; Hokke, C.H.; Deelder, A.M. Discrimination between the anti-monomeric and the anti-multimeric Lewis X response in murine schistosomiasis. Microbes Infect. 2004, 6, 1125–1132. [Google Scholar] [CrossRef]
- van Roon, A.-M.M.; Pannu, N.S.; de Vrind, J.P.M.; van der Marel, G.A.; van Boom, J.H.; Hokke, C.H.; Deelder, A.M.; Abrahams, J.P. Structure of an Anti-Lewis X Fab Fragment in Complex with Its Lewis X Antigen. Structure 2004, 12, 1227–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Remoortere, A.; Vermeer, H.J.; van Roon, A.M.; Langermans, J.A.; Thomas, A.W.; Wilson, R.A.; van Die, I.; van den Eijnden, D.H.; Agoston, K.; Kerekgyarto, J.; et al. Dominant antibody responses to Fuc alpha 1-3GalNAc and Fuc alpha 1-2Fuc alpha 1-3GlcNAc containing carbohydrate epitopes in Pan troglodytes vaccinated and infected with Schistosoma mansoni. Exp. Parasitol. 2003, 105, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Geus, D.C.; van Roon, A.-M.M.; Thomassen, E.A.J.; Hokke, C.H.; Deelder, A.M.; Abrahams, J.P. Characterization of a diagnostic Fab fragment binding trimeric Lewis, X. Proteins Struct. Funct. Bioinform. 2009, 76, 439–447. [Google Scholar] [CrossRef]
- Altman, E.; Harrison, B.A.; Hirama, T.; Chandan, V.; To, R.; MacKenzie, R. Characterization of murine monoclonal antibodies against Helicobacter pylori lipopolysaccharide specific for Lex and Ley blood group determinants. Biochem. Cell Biol. 2005, 83, 589–596. [Google Scholar] [CrossRef]
- Appelmelk, B.J.; Simoons-Smit, I.; Negrini, R.; Moran, A.P.; Aspinall, G.O.; Forte, J.G.; De Vries, T.; Quan, H.; Verboom, T.; Maaskant, J.J.; et al. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect. Immun. 1996, 64, 2031–2040. [Google Scholar] [CrossRef] [Green Version]
- Aspinall, G.O.; Monteiro, M.A.; Shaver, R.T.; Kurjanczyk, L.A.; Penner, J.L. Lipopolysaccharides of Helicobacter Pylori Serogroups O:3 and O:6 Structures of a Class of Lipopolysaccharides with Reference to the Location of Oligomeric Units of D-Glycero-α-D-Manno-Heptose Residues. Eur. J. Biochem. 1997, 248, 592–601. [Google Scholar] [CrossRef]
- Jegatheeswaran, S.; Auzanneau, F.I. Recognition of Lewis X by Anti-Le(x) Monoclonal Antibody IG5F6. J. Immunol. 2019, 203, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Asnani, A.; Auzanneau, F.-I. Synthesis of a BSA-Lex glycoconjugate and recognition of Lex analogues by the anti-Lex monoclonal antibody SH1, The identification of a non-cross reactive analogue. Bioorg. Med. Chem. 2010, 18, 7174–7185. [Google Scholar] [CrossRef]
- Moore, C.J.; Auzanneau, F.I. Understanding the Recognition of Lewis X by Anti-Le Monoclonal Antibodies. J. Med. Chem. 2013, 56, 8183–8190. [Google Scholar] [CrossRef] [Green Version]
- Hendel, J.L.; Auzanneau, F.-I. Convergent Preparation of DimLex Hexasaccharide Analogues. Eur. J. Org. Chem. 2011, 2011, 6864–6876. [Google Scholar] [CrossRef]
- Kamath, V.P.; Diedrich, P.; Hindsgaul, O. Use of diethyl squarate for the coupling of oligosaccharide amines to carrier proteins and characterization of the resulting neoglycoproteins by MALDI-TOF mass spectrometry. Glycoconj. J. 1996, 13, 315–319. [Google Scholar] [CrossRef]
- Leteux, C.; Stoll, M.S.; Childs, R.A.; Chai, W.; Vorozhaikina, M.; Feizi, T. Influence of oligosaccharide presentation on the interactions of carbohydrate sequence-specific antibodies and the selectins—Observations with biotinylated oligosaccharides. J. Immunol. Methods 1999, 227, 109–119. [Google Scholar] [CrossRef]
- Solis, D.; Bruix, M.; Gonzalez, L.; Diaz-Maurino, T.; Rico, M.; Jimenez-Barbero, J.; Feizi, T. Carrier protein-modulated presentation and recognition of an N-glycan: Observations on the interactions of Man(8) glycoform of ribonuclease B with conglutinin. Glycobiology 2001, 11, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solis, D.; Feizi, T.; Yuen, C.T.; Lawson, A.M.; Harrison, R.A.; Loveless, R.W. Differential Recognition by Conglutinin and Mannan-Binding Protein of N-Glycans Presented on Neoglycolipids and Glycoproteins with Special Reference to Complement Glycoprotein C3 and Ribonuclease-B. J. Biol. Chem. 1994, 269, 11555–11562. [Google Scholar] [PubMed]
- Asnani, A.; Auzanneau, F.-I. Synthesis of Lewis X and three Lewis X trisaccharide analogues in which glucose and rhamnose replace N-acetylglucosamine and fucose, respectively. Carbohydr. Res. 2008, 343, 1653–1664. [Google Scholar] [CrossRef]
- Wang, A.; Auzanneau, F.-I. Selective protection of 2-azido-lactose and in situ ferrier rearrangement during glycosylation: Synthesis of a dimeric lewis X fragment. J. Org. Chem. 2007, 72, 3585–3588. [Google Scholar] [CrossRef]
- Wang, A.; Auzanneau, F.-I. Synthesis of LeaLex oligosaccharide fragments and efficient one-step deprotection. Carbohydr. Res. 2010, 345, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Nejatie, A.; Jegatheeswaran, S.; Auzanneau, F.I. Synthesis of LacNAcLex- and DimLex-BSA Conjugates and Binding to Anti-Polymeric Lex mAbs. Eur. J. Org. Chem. 2019, 2019, 6631–6645. [Google Scholar] [CrossRef]
- Forman, A.; Hendel, J.; Auzanneau, F.I. Convergent synthesis of tetra- and penta- saccharide fragments of dimeric Lewis, X. Carbohydr. Res. 2019, 482, 107730. [Google Scholar] [CrossRef]
- Dumon, C.; Bosso, C.; Utille, J.P.; Heyraud, A.; Samain, E. Production of Lewis x Tetrasaccharides by Metabolically Engineered Escherichia coli. ChemBioChem 2006, 7, 359–365. [Google Scholar] [CrossRef]
- Hendel, J.L.; Cheng, A.; Auzanneau, F.-I. Application and limitations of the methyl imidate protection strategy of N-acetylglucosamine for glycosylations at O-4, Synthesis of Lewis A and Lewis X trisaccharide analogues. Carbohydr. Res. 2008, 343, 2914–2923. [Google Scholar] [CrossRef]
- Imberty, A.; Mikros, E.; Koca, J.; Mollicone, R.; Oriol, R.; Pérez, S. Computer simulation of histo-blood group oligosaccharides: Energy maps of all constituting disaccharides and potential energy surfaces of 14 ABH and Lewis carbohydrate antigens. Glycoconj. J. 1995, 12, 331–349. [Google Scholar] [CrossRef]
- Perez, S.; Mouhous-Riou, N.; Nifant’ev, N.E.; Tsvetkov, Y.E.; Bachet, B.; Imberty, A. Crystal and molecular structure of a histo-blood group antigen involved in cell adhesion: The Lewis x trisaccharide. Glycobiology 1996, 6, 537–542. [Google Scholar]
- Reynolds, M.; Fuchs, A.; Lindhorst, T.K.; Perez, S. The hydration features of carbohydrate determinants of Lewis antigens. Mol. Simul. 2008, 34, 447–460. [Google Scholar]
- Miller, K.E.; Mukhopadhyay, C.; Cagas, P.; Bush, C.A. Solution structure of the Lewis x oligosaccharide determined by NMR spectroscopy and molecular dynamics simulations. Biochemistry 1992, 31, 6703–6709. [Google Scholar]
- Haselhorst, T.; Weimar, T.; Peters, T. Molecular Recognition of Sialyl Lewisx and Related Saccharides by Two Lectins. J. Am. Chem. Soc. 2001, 123, 10705–10714. [Google Scholar]
- Bundle, D.R. Antibody Combining Sites and Oligosaccharide Determinants Studied by Competitive-Binding, Sequencing and X-Ray Crystallography. Pure Appl. Chem. 1989, 61, 1171–1180. [Google Scholar]
- Bundle, D.R. Recognition of Carbohydrate Antigens by Antibody Binding Sites. In Biorganic Chemistry: Carbohydrates; Hecht, S.M., Ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Moore, C.J.; Auzanneau, F.-I. Synthesis of 4 manipulated Lewis X trisaccharide analogues. Beilstein J. Org. Chem. 2012, 8, 1134–1143. [Google Scholar]
- Bundle, D.R.; Eichler, E.; Gidney, M.A.J.; Meldal, M.; Ragauskas, A.; Sigurskjold, B.W.; Sinnott, B.; Watson, D.C.; Yaguchi, M.; Young, N.M. Molecular Recognition of a Salmonella Trisaccharide Epitope by Monoclonal-Antibody Se155-4. Biochemistry 1994, 33, 5172–5182. [Google Scholar]
- Yamasaki, R.; Schneider, H.; Griffiss, J.M.; Mandrell, R. Epitope expression of Gonococcal lipooligosaccharide (LOS). Importance of the lipoidal moiety for expression of an epitope that exists in the oligosaccharide moiety of LOS. Mol. Immunol. 1988, 25, 799–809. [Google Scholar]
- Sawen, E.; Hinterholzinger, F.; Landersjo, C.; Widmalm, G. Conformational flexibility of the pentasaccharide LNF-2 deduced from NMR spectroscopy and molecular dynamics simulations. Org. Biomol. Chem. 2012, 10, 4577–4585. [Google Scholar]
- Zaccheus, M.; Pendrill, R.; Jackson, T.A.; Wang, A.; Auzanneau, F.I.; Widmalm, G. Conformational Dynamics of a Central Trisaccharide Fragment of the LeaLex Tumor Associated Antigen Studied by NMR Spectroscopy and Molecular Dynamics Simulations. Eur. J. Org. Chem. 2012, 2012, 4705–4715. [Google Scholar]
- Xia, J.C.; Daly, R.P.; Chuang, F.C.; Parker, L.; Jensen, J.H.; Margulis, C.J. Sugar folding: A novel structural prediction tool for oligosaccharides and polysaccharides. J. Chem. Theory Comput. 2007, 3, 1629–1643. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.C.; Margulis, C. A tool for the prediction of structures of complex sugars. J. Biomol. NMR 2008, 42, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Sawen, E.; Stevensson, B.; Ostervall, J.; Maliniak, A.; Widmalm, G. Molecular Conformations in the Pentasaccharide LNF-1 Derived from NMR Spectroscopy and Molecular Dynamics Simulations. J. Phys. Chem. B 2011, 115, 7109–7121. [Google Scholar] [CrossRef]
- Jackson, T.A.; Robertson, V.; Imberty, A.; Auzanneau, F.-I. The flexibility of the Le(a)Le(x) Tumor Associated Antigen central fragment studied by systematic and stochastic searches as well as dynamic simulations. Bioorg. Med. Chem. 2009, 17, 1514–1526. [Google Scholar] [CrossRef]
- Jackson, T.A.; Robertson, V.; Auzanneau, F.I. Evidence for Two Populated Conformations for the Dimeric Le(X) and Le(A)Le(X) Tumor-Associated Carbohydrate Antigens. J. Med. Chem. 2014, 57, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Quiocho, F.A. Protein-carbohydrate interactions: Basic molecular features. Pure Appl. Chem. 1989, 61, 1293–1306. [Google Scholar] [CrossRef]
- Lemieux, R.U. How Water Provides the Impetus for Molecular Recognition in Aqueous Solution. Acc. Chem. Res. 1996, 29, 373–380. [Google Scholar] [CrossRef]
- Asensio, J.L.; Arda, A.; Canada, F.J.; Jimenez-Barbero, J. Carbohydrate-Aromatic Interactions. Acc. Chem. Res. 2013, 46, 946–954. [Google Scholar] [CrossRef] [Green Version]
- Bundle, D.R. Bioorganic Chemistry: Carbohydrates; Hecht, S.M., Ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Nikrad, P.V.; Beierbeck, H.; Lemieux, R.U. Molecular Recognition 10. A Novel Procedure for the Detection of the Intermolecular Hydrogen-Bonds Present in a Protein-Bullet-Oligosaccharide Complex. Can. J. Chem. 1992, 70, 241–253. [Google Scholar] [CrossRef]
- Van Roon, A.M.M.; Pannu, N.S.; Hokke, C.H.; Deelder, A.M.; JAbrahams, P. Crystallization and preliminary X-ray analysis of an anti-LewisX Fab fragment with and without its LewisX antigen. Acta Crystallogr. D 2003, 59, 1306–1309. [Google Scholar] [CrossRef]









| Entry | Inhibitor | IC50 a (μM) | ∆(∆G) b (kcal·mol−1) |
|---|---|---|---|
| 1 | DimLex (6) | 503 | 0 |
| 2 | Lex (7) | 47 | −1.4 |
| 3 | Lex[1,3]Gal (9) | 529 | 0.03 |
| 4 | GlcNAc[1,3″]Lex (10) | 18637 | 2.1 |
| SH2 | SH1 d | 1G5F6 e | |||
|---|---|---|---|---|---|
| Entry | Inhibitor | IC50 a (μM) | ∆(∆G) b (kcal·mol−1) | ∆(∆G) d (kcal·mol−1) | ∆(∆G) e (kcal·mol−1) |
| 1 | Lex (7) | 47 | 0 | 0 | 0 |
| 2 | LacLex (15) | >>1800 | ---c | 0.2 | 2.5 |
| 3 | GlcLex (16) | >>1800 | ---c | ---c | 2.7 |
| 4 | RhaLex (17) | 827 | 1.7 | 1.1 | 1.6 |
| 5 | RhaLac (18) | >>1800 | ---c | 1.5 | 3.2 |
| 6 | 4″-MeOLex (19) | 148 | 0.7 | ---c | ---c |
| 7 | 4″-HLex (20) | 74 | 0.3 | 1.3 | 0.3 |
| 8 | 4″-ClLex (21) | 19 | -0.5 | 1.6 | 0.3 |
| 9 | 4″-FLex (22) | 17 | -0.6 | 2.1 | −0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jegatheeswaran, S.; Asnani, A.; Forman, A.; Hendel, J.L.; Moore, C.J.; Nejatie, A.; Wang, A.; Wang, J.-W.; Auzanneau, F.-I. Recognition of Dimeric Lewis X by Anti-Dimeric Lex Antibody SH2. Vaccines 2020, 8, 538. https://doi.org/10.3390/vaccines8030538
Jegatheeswaran S, Asnani A, Forman A, Hendel JL, Moore CJ, Nejatie A, Wang A, Wang J-W, Auzanneau F-I. Recognition of Dimeric Lewis X by Anti-Dimeric Lex Antibody SH2. Vaccines. 2020; 8(3):538. https://doi.org/10.3390/vaccines8030538
Chicago/Turabian StyleJegatheeswaran, Sinthuja, Ari Asnani, Adam Forman, Jenifer L. Hendel, Christopher J. Moore, Ali Nejatie, An Wang, Jo-Wen Wang, and France-Isabelle Auzanneau. 2020. "Recognition of Dimeric Lewis X by Anti-Dimeric Lex Antibody SH2" Vaccines 8, no. 3: 538. https://doi.org/10.3390/vaccines8030538
APA StyleJegatheeswaran, S., Asnani, A., Forman, A., Hendel, J. L., Moore, C. J., Nejatie, A., Wang, A., Wang, J. -W., & Auzanneau, F. -I. (2020). Recognition of Dimeric Lewis X by Anti-Dimeric Lex Antibody SH2. Vaccines, 8(3), 538. https://doi.org/10.3390/vaccines8030538