COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of the Papers Included in This Review
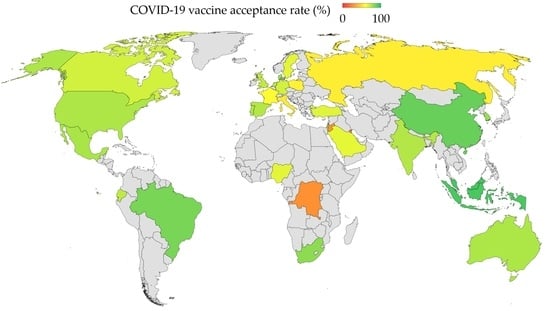
3.2. Rates of COVID-19 Vaccine Acceptance
3.3. Changes in COVID-19 Vaccine Acceptance over Time in Countries with Multiple Survey Studies
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacDonald, N.E.; Sage Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- SAGE Working Group on Vaccine Hesitancy. Report of the SAGE Working Group on Vaccine Hesitancy. Available online: https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf (accessed on 26 December 2020).
- Gowda, C.; Dempsey, A.F. The rise (and fall?) of parental vaccine hesitancy. Hum. Vaccines Immunother. 2013, 9, 1755–1762. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Chandra, R.; Mathur, M.; Samdariya, S.; Kapoor, N. Vaccine hesitancy: Understanding better to address better. Isr. J. Health Policy Res. 2016, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Daley, M.F.; Narwaney, K.J.; Shoup, J.A.; Wagner, N.M.; Glanz, J.M. Addressing Parents’ Vaccine Concerns: A Randomized Trial of a Social Media Intervention. Am. J. Prev. Med. 2018, 55, 44–54. [Google Scholar] [CrossRef]
- Arede, M.; Bravo-Araya, M.; Bouchard, E.; Singh Gill, G.; Plajer, V.; Shehraj, A.; Adam Shuaib, Y. Combating Vaccine Hesitancy: Teaching the Next Generation to Navigate Through the Post Truth Era. Front. Public Health 2018, 6, 381. [Google Scholar] [CrossRef] [Green Version]
- Dube, E.; Vivion, M.; MacDonald, N.E. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: Influence, impact and implications. Expert Rev. Vaccines 2015, 14, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Salmon, D.A.; Dudley, M.Z.; Glanz, J.M.; Omer, S.B. Vaccine Hesitancy: Causes, Consequences, and a Call to Action. Am. J. Prev. Med. 2015, 49, S391–S398. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Cooper, L.Z.; Eskola, J.; Katz, S.L.; Ratzan, S. Addressing the vaccine confidence gap. Lancet 2011, 378, 526–535. [Google Scholar] [CrossRef]
- Olson, O.; Berry, C.; Kumar, N. Addressing Parental Vaccine Hesitancy towards Childhood Vaccines in the United States: A Systematic Literature Review of Communication Interventions and Strategies. Vaccines 2020, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.; MacDonald, N.E.; Marti, M.; Dumolard, L. Vaccine hesitancy around the globe: Analysis of three years of WHO/UNICEF Joint Reporting Form data-2015–2017. Vaccine 2018, 36, 3861–3867. [Google Scholar] [CrossRef]
- Wagner, A.L.; Masters, N.B.; Domek, G.J.; Mathew, J.L.; Sun, X.; Asturias, E.J.; Ren, J.; Huang, Z.; Contreras-Roldan, I.L.; Gebremeskel, B.; et al. Comparisons of Vaccine Hesitancy across Five Low- and Middle-Income Countries. Vaccines 2019, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- The Lancet Child & Adolescent Health. Vaccine hesitancy: A generation at risk. Lancet Child Adolesc. Health 2019, 3, 281. [Google Scholar] [CrossRef]
- Karafillakis, E.; Larson, H.J.; Consortium, A. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine 2017, 35, 4840–4850. [Google Scholar] [CrossRef]
- Pelcic, G.; Karacic, S.; Mikirtichan, G.L.; Kubar, O.I.; Leavitt, F.J.; Cheng-Tek Tai, M.; Morishita, N.; Vuletic, S.; Tomasevic, L. Religious exception for vaccination or religious excuses for avoiding vaccination. Croat. Med. J. 2016, 57, 516–521. [Google Scholar] [CrossRef] [Green Version]
- Yaqub, O.; Castle-Clarke, S.; Sevdalis, N.; Chataway, J. Attitudes to vaccination: A critical review. Soc. Sci. Med. 2014, 112, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, L.C.; Soveri, A.; Lewandowsky, S.; Karlsson, L.; Karlsson, H.; Nolvi, S.; Karukivi, M.; Lindfelt, M.; Antfolk, J. Fearing the disease or the vaccine: The case of COVID-19. Personal. Individ. Differ. 2021, 172, 110590. [Google Scholar] [CrossRef]
- Paul, E.; Steptoe, A.; Fancourt, D. Attitudes towards vaccines and intention to vaccinate against COVID-19: Implications for public health communications. Lancet Reg. Health Eur. 2021, 1. [Google Scholar] [CrossRef]
- Olagoke, A.A.; Olagoke, O.O.; Hughes, A.M. Intention to Vaccinate Against the Novel 2019 Coronavirus Disease: The Role of Health Locus of Control and Religiosity. J. Relig. Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Vallieres, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Pomares, T.D.; Buttenheim, A.M.; Amin, A.B.; Joyce, C.M.; Porter, R.M.; Bednarczyk, R.A.; Omer, S.B. Association of cognitive biases with human papillomavirus vaccine hesitancy: A cross-sectional study. Hum. Vaccines Immunother. 2020, 16, 1018–1023. [Google Scholar] [CrossRef]
- Browne, M.; Thomson, P.; Rockloff, M.J.; Pennycook, G. Going against the Herd: Psychological and Cultural Factors Underlying the ‘Vaccination Confidence Gap’. PLoS ONE 2015, 10, e0132562. [Google Scholar] [CrossRef] [Green Version]
- Hornsey, M.J.; Harris, E.A.; Fielding, K.S. The psychological roots of anti-vaccination attitudes: A 24-nation investigation. Health Psychol. 2018, 37, 307–315. [Google Scholar] [CrossRef]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2021, 9, 16. [Google Scholar] [CrossRef]
- de Figueiredo, A.; Simas, C.; Karafillakis, E.; Paterson, P.; Larson, H.J. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: A large-scale retrospective temporal modelling study. Lancet 2020, 396, 898–908. [Google Scholar] [CrossRef]
- Wellcome Global Monitor. How Does the World Feel about Science and Health? Available online: https://wellcome.org/sites/default/files/wellcome-global-monitor-2018.pdf (accessed on 9 February 2021).
- Larson, H.J.; de Figueiredo, A.; Xiahong, Z.; Schulz, W.S.; Verger, P.; Johnston, I.G.; Cook, A.R.; Jones, N.S. The State of Vaccine Confidence 2016: Global Insights Through a 67-Country Survey. EBioMedicine 2016, 12, 295–301. [Google Scholar] [CrossRef] [Green Version]
- The All-Party Parliamentary Group (APPG) on Vaccinations for All. The Next Decade of Vaccines: Addressing the Challenges That Remain towards Achieving Vaccinations for All. Available online: https://www.results.org.uk/sites/default/files/files/NextDecadeOfVaccines_Single_NoBleed.pdf (accessed on 9 February 2021).
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 10 January 2021).
- World Health Organization. COVID-19 Weekly Epidemiological Update, 22 December 2020. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update---22-december-2020 (accessed on 26 December 2020).
- Prem, K.; Liu, Y.; Russell, T.W.; Kucharski, A.J.; Eggo, R.M.; Davies, N.; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group; Jit, M.; Klepac, P. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: A modelling study. Lancet Public Health 2020, 5, e261–e270. [Google Scholar] [CrossRef] [Green Version]
- Viner, R.M.; Russell, S.J.; Croker, H.; Packer, J.; Ward, J.; Stansfield, C.; Mytton, O.; Bonell, C.; Booy, R. School closure and management practices during coronavirus outbreaks including COVID-19: A rapid systematic review. Lancet Child Adolesc. Health 2020, 4, 397–404. [Google Scholar] [CrossRef]
- Feng, S.; Shen, C.; Xia, N.; Song, W.; Fan, M.; Cowling, B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020, 8, 434–436. [Google Scholar] [CrossRef]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B. Tracking changes in SARS-CoV-2 Spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Hanage, W.P.; Rasmussen, A.L. Making sense of mutation: What D614G means for the COVID-19 pandemic remains unclear. Cell 2020, 182, 794–795. [Google Scholar] [CrossRef]
- Sallam, M.; Ababneh, N.A.; Dababseh, D.; Bakri, F.G.; Mahafzah, A. Temporal increase in D614G mutation of SARS-CoV-2 in the Middle East and North Africa. Heliyon 2021, 7, e06035. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: New Coronavirus Variant Is Identified in UK; British Medical Journal Publishing Group: London, UK, 2020. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Threat Assessment Brief: Rapid Increase of a SARS-CoV-2 Variant with Multiple Spike Protein Mutations Observed in the United Kingdom. Available online: https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-rapid-increase-sars-cov-2-variant-united-kingdom (accessed on 26 December 2020).
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Calina, D.; Docea, A.O.; Petrakis, D.; Egorov, A.M.; Ishmukhametov, A.A.; Gabibov, A.G.; Shtilman, M.I.; Kostoff, R.; Carvalho, F.; Vinceti, M.; et al. Towards effective COVID19 vaccines: Updates, perspectives and challenges (Review). Int. J. Mol. Med. 2020, 46, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Sogni, F.; Affanni, P.; Veronesi, L.; Argentiero, A.; Esposito, S. Vaccines against Coronaviruses: The State of the Art. Vaccines 2020, 8, 309. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Draft Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 26 December 2020).
- Harrison, E.A.; Wu, J.W. Vaccine confidence in the time of COVID-19. Eur. J. Epidemiol. 2020, 35, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Sallam, M.; Dababseh, D.; Eid, H.; Al-Mahzoum, K.; Al-Haidar, A.; Taim, D.; Yaseen, A.; Ababneh, N.A.; Bakri, F.G.; Mahafzah, A. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: A study in Jordan and Kuwait among other Arab countries. Vaccines 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Neumann-Böhme, S.; Varghese, N.E.; Sabat, I.; Barros, P.P.; Brouwer, W.; van Exel, J.; Schreyögg, J.; Stargardt, T. Once We Have It, Will We Use It? A European Survey on Willingness to Be Vaccinated against COVID-19; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Freeman, D.; Loe, B.S.; Chadwick, A.; Vaccari, C.; Waite, F.; Rosebrock, L.; Jenner, L.; Petit, A.; Lewandowsky, S.; Vanderslott, S.; et al. COVID-19 Vaccine Hesitancy in the UK: The Oxford Coronavirus Explanations, Attitudes, and Narratives Survey (OCEANS) II. Psychol. Med. 2020, 1–34. [Google Scholar] [CrossRef]
- Grech, V.; Bonnici, J.; Zammit, D. Vaccine hesitancy in Maltese family physicians and their trainees vis-a-vis influenza and novel COVID-19 vaccination. Early Hum. Dev. 2020, 105259. [Google Scholar] [CrossRef]
- Wang, K.; Wong, E.L.Y.; Ho, K.F.; Cheung, A.W.L.; Chan, E.Y.Y.; Yeoh, E.K.; Wong, S.Y.S. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: A cross-sectional survey. Vaccine 2020, 38, 7049–7056. [Google Scholar] [CrossRef]
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.D.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef]
- Harapan, H.; Wagner, A.L.; Yufika, A.; Winardi, W.; Anwar, S.; Gan, A.K.; Setiawan, A.M.; Rajamoorthy, Y.; Sofyan, H.; Mudatsir, M. Acceptance of a COVID-19 Vaccine in Southeast Asia: A Cross-Sectional Study in Indonesia. Front. Public Health 2020, 8, 381. [Google Scholar] [CrossRef]
- Dror, A.A.; Eisenbach, N.; Taiber, S.; Morozov, N.G.; Mizrachi, M.; Zigron, A.; Srouji, S.; Sela, E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020, 35, 775–779. [Google Scholar] [CrossRef]
- Detoc, M.; Bruel, S.; Frappe, P.; Tardy, B.; Botelho-Nevers, E.; Gagneux-Brunon, A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine 2020, 38, 7002–7006. [Google Scholar] [CrossRef]
- Kwok, K.O.; Li, K.K.; Wei, W.I.; Tang, A.; Wong, S.Y.S.; Lee, S.S. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: A survey. Int. J. Nurs. Stud. 2020, 114, 103854. [Google Scholar] [CrossRef]
- Kabamba Nzaji, M.; Kabamba Ngombe, L.; Ngoie Mwamba, G.; Banza Ndala, D.B.; Mbidi Miema, J.; Luhata Lungoyo, C.; Lora Mwimba, B.; Cikomola Mwana Bene, A.; Mukamba Musenga, E. Acceptability of Vaccination Against COVID-19 Among Healthcare Workers in the Democratic Republic of the Congo. Pragmat. Obs. Res. 2020, 11, 103–109. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Detoc, M.; Bruel, S.; Tardy, B.; Rozaire, O.; Frappe, P.; Botelho-Nevers, E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: A cross sectional survey. J. Hosp. Infect. 2020. [Google Scholar] [CrossRef]
- Sarasty, O.; Carpio, C.E.; Hudson, D.; Guerrero-Ochoa, P.A.; Borja, I. The demand for a COVID-19 vaccine in Ecuador. Vaccine 2020, 38, 8090–8098. [Google Scholar] [CrossRef]
- Wong, L.P.; Alias, H.; Wong, P.F.; Lee, H.Y.; AbuBakar, S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum. Vaccines Immunother. 2020, 16, 2204–2214. [Google Scholar] [CrossRef]
- Ward, J.K.; Alleaume, C.; Peretti-Watel, P.; Group, C. The French public’s attitudes to a future COVID-19 vaccine: The politicization of a public health issue. Soc. Sci. Med. 2020, 265, 113414. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes Toward a Potential SARS-CoV-2 Vaccine: A Survey of U.S. Adults. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef]
- Salali, G.D.; Uysal, M.S. COVID-19 vaccine hesitancy is associated with beliefs on the origin of the novel coronavirus in the UK and Turkey. Psychol. Med. 2020, 1–3. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Z.; Zhao, Q.; Alias, H.; Danaee, M.; Wong, L.P. Understanding COVID-19 vaccine demand and hesitancy: A nationwide online survey in China. PLoS Negl. Trop. Dis. 2020, 14, e0008961. [Google Scholar] [CrossRef]
- Taylor, S.; Landry, C.A.; Paluszek, M.M.; Groenewoud, R.; Rachor, G.S.; Asmundson, G.J.G. A Proactive Approach for Managing COVID-19: The Importance of Understanding the Motivational Roots of Vaccination Hesitancy for SARS-CoV2. Front. Psychol. 2020, 11, 575950. [Google Scholar] [CrossRef]
- Reiter, P.L.; Pennell, M.L.; Katz, M.L. Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine 2020, 38, 6500–6507. [Google Scholar] [CrossRef]
- Malik, A.A.; McFadden, S.M.; Elharake, J.; Omer, S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 2020, 26, 100495. [Google Scholar] [CrossRef]
- Barello, S.; Nania, T.; Dellafiore, F.; Graffigna, G.; Caruso, R. ‘Vaccine hesitancy’ among university students in Italy during the COVID-19 pandemic. Eur. J. Epidemiol. 2020, 35, 781–783. [Google Scholar] [CrossRef]
- Rhodes, A.; Hoq, M.; Measey, M.A.; Danchin, M. Intention to vaccinate against COVID-19 in Australia. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Bell, S.; Clarke, R.; Mounier-Jack, S.; Walker, J.L.; Paterson, P. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: A multi-methods study in England. Vaccine 2020, 38, 7789–7798. [Google Scholar] [CrossRef]
- Sherman, S.M.; Smith, L.E.; Sim, J.; Amlot, R.; Cutts, M.; Dasch, H.; Rubin, G.J.; Sevdalis, N. COVID-19 vaccination intention in the UK: Results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum. Vaccines Immunother. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.C.; Fang, Y.; Cao, H.; Chen, H.; Hu, T.; Chen, Y.Q.; Zhou, X.; Wang, Z. Parental acceptability of COVID-19 vaccination for children under the age of 18 years in China: Cross-sectional online survey. JMIR Pediatr. Parent. 2020. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Negri, E.; Alicandro, G.; Scarpino, V. Attitudes towards influenza vaccine and a potential COVID-19 vaccine in Italy and differences across occupational groups, September 2020. Med. Lav. 2020, 111, 445–448. [Google Scholar] [CrossRef]
- Grech, V.; Gauci, C.; Agius, S. Vaccine hesitancy among Maltese healthcare workers toward influenza and novel COVID-19 vaccination. Early Hum. Dev. 2020, 105213. [Google Scholar] [CrossRef]
- Grech, V.; Gauci, C. Vaccine hesitancy in the University of Malta Faculties of Health Sciences, Dentistry and Medicine vis-a-vis influenza and novel COVID-19 vaccination. Early Hum. Dev. 2020, 105258. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohaithef, M.; Padhi, B.K. Determinants of COVID-19 Vaccine Acceptance in Saudi Arabia: A Web-Based National Survey. J. Multidiscip. Healthc. 2020, 13, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Phadke, V.K.; Bednarczyk, R.A.; Salmon, D.A.; Omer, S.B. Association between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis. JAMA 2016, 315, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benecke, O.; DeYoung, S.E. Anti-Vaccine Decision-Making and Measles Resurgence in the United States. Glob. Pediatr. Health 2019, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangarosa, E.J.; Galazka, A.M.; Wolfe, C.R.; Phillips, L.M.; Gangarosa, R.E.; Miller, E.; Chen, R.T. Impact of anti-vaccine movements on pertussis control: The untold story. Lancet 1998, 351, 356–361. [Google Scholar] [CrossRef]
- Borba, R.C.; Vidal, V.M.; Moreira, L.O. The re-emergency and persistence of vaccine preventable diseases. An. Acad. Bras. Cienc. 2015, 87, 1311–1322. [Google Scholar] [CrossRef] [Green Version]
- Wong, L.P.; Wong, P.F.; AbuBakar, S. Vaccine hesitancy and the resurgence of vaccine preventable diseases: The way forward for Malaysia, a Southeast Asian country. Hum. Vaccines Immunother. 2020, 16, 1511–1520. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Graham, B.S. Rapid COVID-19 vaccine development. Science 2020, 368, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Pogue, K.; Jensen, J.L.; Stancil, C.K.; Ferguson, D.G.; Hughes, S.J.; Mello, E.J.; Burgess, R.; Berges, B.K.; Quaye, A.; Poole, B.D. Influences on Attitudes Regarding Potential COVID-19 Vaccination in the United States. Vaccines 2020, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, J.D.; Hasan, M.I.; Baldi, A.J.; Hossain, S.J.; Shiraji, S.; Bhuiyan, M.S.A.; Mehrin, S.F.; Fisher, J.; Tofail, F.; Tipu, S.M.U. Immediate impact of stay-at-home orders to control COVID-19 transmission on socioeconomic conditions, food insecurity, mental health, and intimate partner violence in Bangladeshi women and their families: An interrupted time series. Lancet Glob. Health 2020, 8, e1380–e1389. [Google Scholar] [CrossRef]
- Ridenhour, B.; Kowalik, J.M.; Shay, D.K. Unraveling r 0: Considerations for public health applications. Am. J. Public Health 2018, 108, S445–S454. [Google Scholar] [CrossRef]
- Billah, M.A.; Miah, M.M.; Khan, M.N. Reproductive number of coronavirus: A systematic review and meta-analysis based on global level evidence. PLoS ONE 2020, 15, e0242128. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Vegvari, C.; Truscott, J.; Collyer, B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 2020, 396, 1614–1616. [Google Scholar] [CrossRef]
- Britton, T.; Ball, F.; Trapman, P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science 2020, 369, 846–849. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O., 3rd. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS Pharm. Sci. Tech. 2020, 21, 225. [Google Scholar] [CrossRef]
- Teerawattananon, Y.; Dabak, S.V. COVID Vaccination Logistics: Five Steps to Take Now; Nature Publishing Group: London, UK, 2020. [Google Scholar]
- Palamenghi, L.; Barello, S.; Boccia, S.; Graffigna, G. Mistrust in biomedical research and vaccine hesitancy: The forefront challenge in the battle against COVID-19 in Italy. Eur. J. Epidemiol. 2020, 35, 785–788. [Google Scholar] [CrossRef]
- Weintraub, R.L.; Subramanian, L.; Karlage, A.; Ahmad, I.; Rosenberg, J. COVID-19 Vaccine To Vaccination: Why Leaders Must Invest In Delivery Strategies Now: Analysis describe lessons learned from past pandemics and vaccine campaigns about the path to successful vaccine delivery for COVID-19. Health Aff. 2021, 40, 33–41. [Google Scholar] [CrossRef]
- Habersaat, K.B.; Betsch, C.; Danchin, M.; Sunstein, C.R.; Böhm, R.; Falk, A.; Brewer, N.T.; Omer, S.B.; Scherzer, M.; Sah, S. Ten considerations for effectively managing the COVID-19 transition. Nat. Hum. Behav. 2020, 4, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R.H.; Cvejic, E.; Bonner, C.; Pickles, K.; McCaffery, K.J.; Sydney Health Literacy Lab COVID-19 Group. Willingness to vaccinate against COVID-19 in Australia. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Shaukat, N.; Ali, D.M.; Razzak, J. Physical and mental health impacts of COVID-19 on healthcare workers: A scoping review. Int. J. Emerg. Med. 2020, 13, 40. [Google Scholar] [CrossRef]
- Nie, Q.; Li, X.; Chen, W.; Liu, D.; Chen, Y.; Li, H.; Li, D.; Tian, M.; Tan, W.; Zai, J. Phylogenetic and phylodynamic analyses of SARS-CoV-2. Virus Res. 2020, 287, 198098. [Google Scholar] [CrossRef]
- Sallam, M.; Dababseh, D.; Yaseen, A.; Al-Haidar, A.; Taim, D.; Eid, H.; Ababneh, N.A.; Bakri, F.G.; Mahafzah, A. COVID-19 misinformation: Mere harmless delusions or much more? A knowledge and attitude cross-sectional study among the general public residing in Jordan. PLoS ONE 2020, 15, e0243264. [Google Scholar] [CrossRef]
- Nyhan, B.; Zeitzoff, T. Conspiracy and misperception belief in the Middle East and North Africa. J. Politics 2018, 80, 1400–1404. [Google Scholar] [CrossRef] [Green Version]
- Sallam, M.; Dababseh, D.; Yaseen, A.; Al-Haidar, A.; Ababneh, N.A.; Bakri, F.G.; Mahafzah, A. Conspiracy Beliefs Are Associated with Lower Knowledge and Higher Anxiety Levels Regarding COVID-19 among Students at the University of Jordan. Int. J. Environ. Res. Public Health 2020, 17, 4915. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Danladi, M.M.A.; Saleh, D.A.; Ejembi, P.E. Knowledge, Attitudes and Practices Towards COVID-19: An Epidemiological Survey in North-Central Nigeria. J. Community Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Head, K.J.; Kasting, M.L.; Sturm, L.A.; Hartsock, J.A.; Zimet, G.D. A National Survey Assessing SARS-CoV-2 Vaccination Intentions: Implications for Future Public Health Communication Efforts. Sci. Commun. 2020, 42, 698–723. [Google Scholar] [CrossRef]


| Study | Country | Date of Survey | Response Recorded as Vaccine Acceptance | N 6 | Target Population | Acceptance Rate (%) | Age/Sex Correlation with Higher Vaccine Acceptance |
|---|---|---|---|---|---|---|---|
| Wang et al. [50] | Hong Kong | February and March, 2020 | Intend to accept | 806 | Nurses | 40.0 | Male |
| Wang et al. [51] | China | March, 2020 | Yes | 2058 | General population | 91.3 | Male |
| Harapan et al. [52] | Indonesia | March and April 2020 | Yes | 1359 | General population | 93.3 | None |
| Dror et al. [53] | Israel | March and April 2020 | Yes | 388 | Doctors | 78.1 | - |
| Detoc et al. [54] | France | March and April 2020 | Yes certainly/possibly | 3259 | General population | 77.6 | Male, age |
| Dror et al. [53] | Israel | March and April 2020 | Yes | 1112 | General population | 75.0 | - |
| Kwok et al. [55] | Hong Kong | March and April 2020 | Likely to vaccinate (scored 6 or above out of 10) | 1205 | Nurses | 63.0 | Age |
| Dror et al. [53] | Israel | March and April 2020 | Yes | 211 | Nurses | 61.1 | - |
| Nzaji et al. [56] | DRC 2 | March and April 2020 | Yes | 613 | Healthcare workers | 27.7 | Age |
| Gagneux-Brunon et al. [57] | France | March to July, 2020 | Yes | 2047 | Healthcare workers | 76.9 | Male, age |
| Sarasty et al. [58] | Ecuador | April, 2020 | Willing to accept a vaccine | 1050 | General population | 97.0 | - |
| Wong et al. [59] | Malaysia | April, 2020 | Definitely, probably or possibly yes | 1159 | General population | 94.3 | Male |
| Neumann-Böhme et al. [47] 1 | Denmark | April, 2020 | Yes | 1000 | General population | 80.0 | - |
| Neumann-Böhme et al. [47] | UK 3 | April, 2020 | Yes | 1000 | General population | 79.0 | - |
| Neumann-Böhme et al. [47] | Italy | April, 2020 | Yes | 1500 | General population | 77.3 | - |
| Ward et al. [60] | France | April and May 2020 | Certainly or probably | 5018 | General population | 76.0 | None |
| Neumann-Böhme et al. [47] | Portugal | April, 2020 | Yes | 1000 | General population | 75.0 | - |
| Neumann-Böhme et al. [47] | Netherland | April, 2020 | Yes | 1000 | General population | 73.0 | - |
| Neumann-Böhme et al. [47] | Germany | April, 2020 | Yes | 1000 | General population | 70.0 | - |
| Neumann-Böhme et al. [47] | France | April, 2020 | Yes | 1000 | General population | 62.0 | - |
| Fisher et al. [61] | US 4 | April, 2020 | Yes | 1003 | General population | 56.9 | Male, age |
| Salali & Uysal [62] | UK | May, 2020 | Yes | 1088 | General population | 83.0 | None |
| Lin et al. [63] | China | May, 2020 | Definitely/probably yes | 3541 | General population | 83.5 | None |
| Taylor et al. [64] | Canada | May, 2020 | Yes | 1902 | General population | 80.0 | Male, age |
| Taylor et al. [64] | US | May, 2020 | Yes | 1772 | General population | 75.0 | Male, age |
| Salali & Uysal [62] | Turkey | May, 2020 | Yes | 3936 | General population | 66.0 | Male |
| Reiter et al. [65] | US | May, 2020 | Definitely/probably willing | 2006 | General population | 68.5 | Male |
| Malik et al. [66] | US | May, 2020 | Agree/strongly agree | 672 | General population | 67.0 | Male, age |
| Lazarus et al. [46] | China | June, 2020 | Completely/somewhat agree | 712 | General population | 88.6 | - |
| Barello et al. [67] | Italy | June, 2020 | Yes | 735 | University students | 86.1 | - |
| Lazarus et al. [46] | Brazil | June, 2020 | Completely/somewhat agree | 717 | General population | 85.4 | - |
| Lazarus et al. [46] | South Africa | June, 2020 | Completely/somewhat agree | 619 | General population | 81.6 | - |
| Lazarus et al. [46] | South Korea | June, 2020 | Completely/somewhat agree | 752 | General population | 79.8 | - |
| Lazarus et al. [46] | Mexico | June, 2020 | Completely/somewhat agree | 699 | General population | 76.3 | - |
| Lazarus et al. [46] | US | June, 2020 | Completely/somewhat agree | 773 | General population | 75.4 | - |
| Lazarus et al. [46] | India | June, 2020 | Completely/somewhat agree | 742 | General population | 74.5 | - |
| Lazarus et al. [46] | Spain | June, 2020 | Completely/somewhat agree | 748 | General population | 74.3 | - |
| Lazarus et al. [46] | Ecuador | June, 2020 | Completely/somewhat agree | 741 | General population | 71.9 | - |
| Lazarus et al. [46] | UK | June, 2020 | Completely/somewhat agree | 768 | General population | 71.5 | - |
| Lazarus et al. [46] | Italy | June, 2020 | Completely/somewhat agree | 736 | General population | 70.8 | - |
| Lazarus et al. [46] | Canada | June, 2020 | Completely/somewhat agree | 707 | General population | 68.7 | - |
| Lazarus et al. [46] | Germany | June, 2020 | Completely/somewhat agree | 722 | General population | 68.4 | - |
| Lazarus et al. [46] | Singapore | June, 2020 | Completely/somewhat agree | 655 | General population | 67.9 | - |
| Lazarus et al. [46] | Sweden | June, 2020 | Completely/somewhat agree | 650 | General population | 65.2 | - |
| Lazarus et al. [46] | Nigeria | June, 2020 | Completely/somewhat agree | 670 | General population | 65.2 | - |
| Lazarus et al. [46] | France | June, 2020 | Completely/somewhat agree | 669 | General population | 58.9 | - |
| Lazarus et al. [46] | Poland | June, 2020 | Completely/somewhat agree | 666 | General population | 56.3 | - |
| Lazarus et al. [46] | Russia | June, 2020 | Completely/somewhat agree | 680 | General population | 54.9 | - |
| Rhodes et al. [68] | Australia | June, 2020 | Yes | 2018 | Parents and guardians | 75.8 | Male, age |
| Bell et al. [69] | UK | July, 2020 | Yes, definitely or unsure but leaning towards yes | 1252 | Parents and guardians | 89.1 | - |
| Sherman et al. [70] | UK | July, 2020 | Very likely | 1500 | General population | 64.0 | Age |
| Zhang et al. [71] | China | September, 2020 | Likely or very likely | 1052 | Parents and guardians | 72.6 | None |
| Gretch et al. [49] | Malta | September, 2020 | Likely | 123 | GPs and GP trainees | 61.8 | - |
| La Vecchia et al. [72] | Italy | September, 2020 | Yes/probably yes | 1055 | General population | 53.7 | - |
| Gretch et al. [73] | Malta | September, 2020 | Likely | 1002 | Healthcare workers | 52.0 | - |
| Gretch & Gauci [74] | Malta | September, 2020 | Likely | 852 | University students/staff | 44.2 | - |
| Freeman et al. [48] | UK | September and October, 2020 | Endorsing 4/7 items of Oxford Scale 5 | 5114 | General population | 71.7 | Male, age |
| Al-Mohaithef & Badhi [75] | Saudi Arabia | Unknown | Yes | 992 | General population | 64.7 | None |
| Sallam et al. [45] | Jordan | December, 2020 | Yes | 2173 | General population | 28.4 | Male |
| Sallam et al. [45] | Kuwait | December, 2020 | Yes | 771 | General population | 23.6 | Male |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. https://doi.org/10.3390/vaccines9020160
Sallam M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines. 2021; 9(2):160. https://doi.org/10.3390/vaccines9020160
Chicago/Turabian StyleSallam, Malik. 2021. "COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates" Vaccines 9, no. 2: 160. https://doi.org/10.3390/vaccines9020160
APA StyleSallam, M. (2021). COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines, 9(2), 160. https://doi.org/10.3390/vaccines9020160