Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Characterizations
2.3. MD Integrated with Crystallization Experiments
2.4. MD Integrated with Crystallization Characterizations
3. Results and Discussion
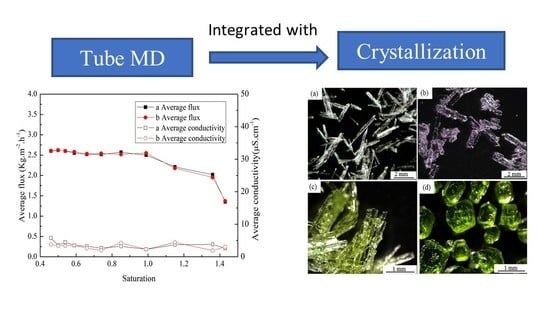
3.1. Single System Crystallization by MD Integrated with Crystallization
3.2. Preferential Crystallization of Zinc Sulfate by MD Integrated with Crystallization
3.3. Preferential Crystallization of Nickel Sulfate by MD Integrated with Crystallization
3.4. Co-Crystallization of Zinc and Nickel Sulfate by MD Integrated with Crystallization
3.5. Membrane Fouling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chitpong, N.; Husson, S.M. Nanofiber ion-exchange membranes for the rapid uptake and recovery of heavy metals from water. Membranes 2016, 6, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, W.; Qadir, S.; Ahmad, T. Synthesis and characterization of molecular imprinted nanomaterials for the removal of heavy metals from water. J. Mater. Res. Technol. 2017, 7, 270–282. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, W.; Ahmad, T. Original article Synthesis and characterization of molecularly imprinted magnetite nanomaterials as a novel adsorbent for the removal of heavy metals from aqueous solution. J. Mater. Res. Technol. 2019, 8, 4239–4252. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Memb. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- García, J.V.; Dow, N.; Milne, N.; Zhang, J.; Naidoo, L.; Gray, S.; Duke, M. Membrane distillation trial on textile wastewater containing surfactants using hydrophobic and hydrophilic-coated polytetrafluoroethylene (PTFE) membranes. Membranes 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Ashoor, B.B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S.W. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Memb. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Ulrich, J.; Frohberg, P. Problems, potentials and future of industrial crystallization. Front. Chem. Sci. Eng. 2013, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Curcio, E.; Drioli, E. Membrane distillation and related operations—A review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Gryta, M. Concentration of NaCl solution by membrane distillation integrated with crystallization. Sep. Sci. Technol. 2002, 37, 3535–3558. [Google Scholar] [CrossRef]
- Yuan, N.; Ji, Z.; Yang, L.; Xu, Z.; Li, Y.; Wang, W. Treatment of Multivariate Heavy Metal Solution by Membrane Distillation Based on PTFE Hollow Fiber Membrane. Chin. J. Rare Met. 2017. [Google Scholar] [CrossRef]
- Gryta, M. Direct contact membrane distillation with crystallization applied to NaCl solutions. Chem. Pap. Acad. Sci. 2002, 56, 14–19. [Google Scholar]
- Drioli, E.; Curcio, E.; Criscuoli, A.; Di Profio, G. Integrated system for recovery of CaCO3, NaCl and MgSO4·7H2O from nanofiltration retentate. J. Memb. Sci. 2004, 239, 27–38. [Google Scholar] [CrossRef]
- Edwie, F.; Chung, T.-S. Development of hollow fiber membranes for water and salt recovery from highly concentrated brine via direct contact membrane distillation and crystallization. J. Memb. Sci. 2012, 421, 111–123. [Google Scholar] [CrossRef]
- Edwie, F.; Chung, T.-S. Development of simultaneous membrane distillation–crystallization (smembrane distillation integrated with crystallization) technology for treatment of saturated brine. Chem. Eng. Sci. 2013, 98, 160–172. [Google Scholar] [CrossRef]
- Chabanon, E.; Mangin, D.; Charcosset, C. Membranes and crystallization processes: State of the art and prospects. J. Memb. Sci. 2016, 509, 57–67. [Google Scholar] [CrossRef]
- Tun, C.M.; Fane, A.G.; Matheickal, J.T.; Sheikholeslami, R. Membrane distillation crystallization of concentrated salts—Flux and crystal formation. J. Memb. Sci. 2005, 257, 144–155. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Lim, S.Y.; Liang, Y.Y.; Weihs, G.A.F.; Wiley, D.E.; Fletcher, D.F. A CFD study on the effect of membrane permeance on permeate flux enhancement generated by unsteady slip velocity. J. Memb. Sci. 2018, 556, 138–145. [Google Scholar] [CrossRef]
- Razzaghi, M.H.; Tavakolmoghadam, M.; Rekabdar, F.; Oveisi, F. Investigation of the effect of coagulation bath composition on PVDF/CA membrane by evaluating critical flux and antifouling properties in lab-scale submerged MBR. Water Environ. J. 2018, 32, 366–376. [Google Scholar] [CrossRef]
- Khan, E.U.; Nordberg, Å. Membrane distillation process for concentration of nutrients and water recovery from digestate reject water. Sep. Purif. Technol. 2018, 206, 90–98. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Hong, S. Recovery of water and minerals from shale gas produced water by membrane distillation crystallization. Water Res. 2018, 129, 447–459. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Kadir, S.H.S.A.; Jamalludin, M.R.; Harun, Z.; Aziz, M.H.A.; Rahman, M.A.; Jaafar, J.; Nomura, M.; Honda, S. Removal of As (III) and As (V) from water using green, silica-based ceramic hollow fibre membranes via direct contact membrane distillation. RSC Adv. 2019, 9, 3367–3376. [Google Scholar] [CrossRef] [Green Version]
- Barieau, R.E.; Giauque, W.F. ZnSO4·7H2O. ZnSO4·6H2O. Heat Capacities, Entropies and Crystal Perfection at Low Temperatures. Heats of Solution and Transition. J. Am. Chem. Soc. 1950, 72, 5676–5684. [Google Scholar] [CrossRef]
- Krishnamurti, D. The Raman spectra of crystalline sulphates of Ni and Mn. Proc. Indian Acad. 1958, 48, 355–363. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Wang, Y.; Zhang, M.; Xiao, Q.; Duan, Y. Influential analysis of concentration polarization on water flux and power density in PRO process: Modeling and experiments. Desalination 2017, 412, 39–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Hou, L.; Kuang, S.; Yu, A. Modeling of the variations of permeate flux, concentration polarization, and solute rejection in nanofiltration system. AIChE J. 2019, 65, 1076–1087. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Porter, C.J.; Cheng, W.; Zhang, X.; Wang, L.; Elimelech, M. Concentration and Recovery of Dyes from Textile Wastewater Using a Self-Standing, Support-Free Forward Osmosis Membrane. Environ. Sci. Technol. 2019, 53, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, S. Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Memb. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Wang, Z.; Jin, J.; Lin, S. Novel Janus membrane for membrane distillation with simultaneous fouling and wetting resistance. Environ. Sci. Technol. 2017, 51, 13304–13310. [Google Scholar] [CrossRef]











| Material | Temperature/°C | Solubility | Mass Fraction/% |
|---|---|---|---|
| ZnSO4 | 10 | 47.20 | 32.07 |
| 65 | 74.33 | 42.63 | |
| NiSO4 | 10 | 32.00 | 24.24 |
| 65 | 57.83 | 36.64 |
| Ratios of Zinc Sulfate and Nickle Sulfate | Method |
|---|---|
| 3:1 | Preferential crystallization of Zinc sulfate |
| 0.5:1 | Preferential crystallization of Nickle sulfate |
| 1.5:1 | Mixed co-crystallization |
| Membrane and Module | Properties |
|---|---|
| Diameter/mm | 22 |
| Membrane thickness/mm | 0.300 |
| Nominal pore size/µm | 0.083 |
| Maximum pore size/µm | 0.211 |
| Contact angle/deg | 115.7 |
| Tensile stress at break/MPa | 54 |
| Strain at break/% | 240 |
| Effective length/m | 0.308 |
| Effective membrane area/(m2) | 0.213 |
| Number | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Composition of the treated solutions | New membrane | Zinc sulfate | Nickel sulfate | Zinc and nickel sulfate |
| Work time | 0 h | 515 h | 22 h | 95 h |
| Membrane Number/Element Content % | C | O | F | S | Zn | Ni |
|---|---|---|---|---|---|---|
| 0 | 23.33 | - | 76.67 | - | - | 0 |
| 1 | 24.39 | - | 73.63 | 0.32 | 3.86 | - |
| 2 | 19.99 | - | 80.01 | - | - | - |
| 3 | 24.99 | 6.15 | 56.58 | - | 11.2 | 1.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, X.-Y.; Xu, Z.; Bai, A.-P.; Resina-Gallego, M.; Ji, Z.-G. Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization. Membranes 2020, 10, 19. https://doi.org/10.3390/membranes10010019
Lou X-Y, Xu Z, Bai A-P, Resina-Gallego M, Ji Z-G. Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization. Membranes. 2020; 10(1):19. https://doi.org/10.3390/membranes10010019
Chicago/Turabian StyleLou, Xiang-Yang, Zheng Xu, An-Ping Bai, Montserrat Resina-Gallego, and Zhong-Guang Ji. 2020. "Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization" Membranes 10, no. 1: 19. https://doi.org/10.3390/membranes10010019
APA StyleLou, X. -Y., Xu, Z., Bai, A. -P., Resina-Gallego, M., & Ji, Z. -G. (2020). Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization. Membranes, 10(1), 19. https://doi.org/10.3390/membranes10010019