Sorption/Diffusion Contributions to the Gas Permeation Properties of Bi-Soft Segment Polyurethane/Polycaprolactone Membranes for Membrane Blood Oxygenators
Abstract
:1. Introduction
2. Theory
2.1. Solution–Diffusion Model
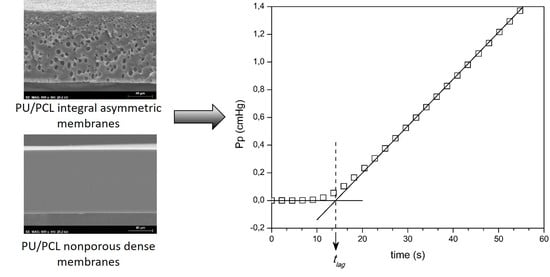
2.2. Diffusion in Transient State: Time–Lag Method
3. Materials and Methods
3.1. Materials
3.2. Membrane Synthesis
3.3. Scanning Electron Microscopy
3.4. Gas Permeation by the Constant Volume Method
3.4.1. Experimental Setup
3.4.2. Procedure
3.5. Gas Solubility by the Barometric Method
3.6. Statistical Analysis
4. Results and Discussion
4.1. SEM
4.2. Permeation of CO2, O2, and N2 through Nonporous Symmetric PU Membranes (Group 1)
4.3. Determination of the CO2, O2, and N2 Diffusion and Solubility Coefficients of the Nonporous Symmetric PU Membranes by the Time-Lag Method
4.4. CO2, O2, and N2 Permeation of the Integral Asymmetric PU Membranes from Group 2
4.5. Determination of the CO2, O2, and N2 Solubility Coefficient for a Test Membrane by the Barometric Method
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



References
- Gravlee, G.P.; Davis, R.F.; Utley, J.R. Cardiopulmonary Bypass: Principles and Practice; Williams & Wilkins: Baltimore, MD, USA, 1993. [Google Scholar]
- Nanda, N.C.; Trehan, N.; Airan, B.; Conrad, S.A.; Mehta, Y. Manual of Extracorporeal Membrane Oxygenation (ECMO) in the ICU; Jaypee Brothers Med. Publ.: New Delhi, India, 2014. [Google Scholar]
- Federspiel, W.J.; Henchir, K.A. Lung Artificial: Basic Principles and Current Applications. Encycl. Biomater. Biomed. Eng. 2004, 9, 910–921. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Papenburg, B.J.; Gironés, M.; Saiful, S.; Bettahalli, S.N.M.; Schmitmeier, S.; Wessling, M. Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. J. Membr. Sci. 2008, 308, 1–34. [Google Scholar] [CrossRef] [Green Version]
- De Somer, F. Does contemporary oxygenator design influence haemolysis? Perfusion 2013, 28, 280–285. [Google Scholar] [CrossRef]
- Melchior, R.; Sutton, S.W.; Harris, W.; Dalton, H.J. Evolution of membrane oxygenator technology for utilization during pediatric cardiopulmonary bypass, Pediatr. Health Med. Ther. 2016, 7, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Yeager, T.; Roy, S. Evolution of Gas Permeable Membranes for Extracorporeal Membrane Oxygenation. Artif. Organs 2017, 41, 700–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwahashi, H.; Yuri, K.; Nosé, Y. Development of the oxygenator: Past, present, and future. J. Artif. Organs 2004, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zdrahala, R.J.; Zdrahala, I.J. Biomedical Applications of Polyurethanes: A Review of Past Promises, Present Realities, and a Vibrant Future. J. Biomater. Appl. 1999, 14, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Tersac, G. Chemistry and technology of polyols for polyurethanes. Milhail Ionescu. Rapra Technology, Shrewsbury, UK. Polym. Int. 2007, 56, 820. [Google Scholar] [CrossRef]
- Boretos, J.W.; Pierce, W.S. Segmented Polyurethane: A New Elastomer for Biomedical Applications. Science 1967, 158, 1481–1482. [Google Scholar] [CrossRef]
- Marzec, M.; Kucińska-Lipka, J.; Kalaszczyńska, I.; Janik, H. Development of polyurethanes for bone repair. Mater. Sci. Eng. C 2017, 80, 736–747. [Google Scholar] [CrossRef]
- Plastics in Medical Devices for Cardiovascular Applications, 1st ed. Available online: https://www.elsevier.com/books/plastics-in-medical-devices-for-cardiovascular-applications/padsalgikar/978-0-323-35885-9 (accessed on 4 December 2018).
- Zhao, C.; de Pinho, M.N.N. Design of polypropylene oxide/polybutadiene bi-soft segment urethane/urea polymer for pervaporation membranes. Polymer 1999, 40, 6089–6097. [Google Scholar] [CrossRef]
- Queiroz, D.P.; Pinho, M.N. Gas permeability of polypropylene oxide/polybutadiene bi-soft segment urethane/urea membranes. Science 2002, 145, 379–383. [Google Scholar] [CrossRef]
- Queiroz, D.P.; de Pinho, M.N.; Dias, C. ATR−FTIR Studies of Poly(propylene oxide)/Polybutadiene Bi-Soft Segment Urethane/Urea Membranes. Macromolecules 2003, 36, 4195–4200. [Google Scholar] [CrossRef]
- Queiroz, D.P.; de Pinho, M.N. Structural characteristics and gas permeation properties of polydimethylsiloxane/poly(propylene oxide) urethane/urea bi-soft segment membranes. Polymer 2005, 46, 2346–2353. [Google Scholar] [CrossRef]
- Queiroz, D.P.; Pinto, I.M.; Besteiro, M.C.F.; Silva, A.F.M.; Gil, M.H.; Guiomar, A.J.; de Pinho, M.N. Surface and hemocompatibility studies of bi-soft segment polyurethane membranes. Int. J. Artif. Organs 2006, 29, 866–872. [Google Scholar] [CrossRef]
- Shin’oka, T.; Matsumura, G.; Hibino, N.; Naito, Y.; Watanabe, M.; Konuma, T.; Sakamoto, T.; Nagatsu, M.; Kurosawa, H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 2005, 129, 1330–1338. [Google Scholar] [CrossRef] [Green Version]
- Besteiro, M.C.; Guiomar, A.J.; Gonçalves, C.A.; Bairos, V.A.; de Pinho, M.N.; Gil, M.H. Characterization and in vitro hemocompatibility of bi-soft segment, polycaprolactone-based poly(ester urethane urea) membranes. J. Biomed. Mater. Res. Part A 2009, 93, 954–964. [Google Scholar] [CrossRef]
- Faria, M.; de Pinho, M.N. Phase segregation and gas permeation properties of poly(urethane urea) bi-soft segment membranes. Eur. Polym. J. 2016, 82, 260–276. [Google Scholar] [CrossRef]
- Faria, M.; Rajagopalan, M.; de Pinho, M.N. Tailoring bi-soft segment poly(ester urethane urea) integral asymmetric membranes for CO2 and O2 permeation. J. Membr. Sci. 2012, 387–388, 66–75. [Google Scholar] [CrossRef]
- De Pinho, M.N. Process of Synthesis Asymmetric Polyurethane Based Membranes with Hemocompatibility Characteristics and Membranes Obtained by Said Process. U.S. Patent 2010/0111761 A1, 6 May 2010. [Google Scholar]
- Faria, M.; Brogueira, P.; de Pinho, M.N. Sub-micron tailoring of bi-soft segment asymmetric polyurethane membrane surfaces with enhanced hemocompatibility properties. Colloids Surf. B Biointerfaces 2011, 86, 21–27. [Google Scholar] [CrossRef]
- Faria, M.; Geraldes, V.; de Pinho, M.N. Surface characterization of asymmetric Bi-soft segment poly(ester urethane urea) membranes for blood-oxygenation medical devices. Int. J. Biomater. 2012, 2012, 376321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.F.; Khulbe, K.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; Available online: https://www.springer.com/gp/book/9783319010946 (accessed on 5 December 2018).
- Mulder, J. Basic Principles of Membrane Technology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Barrer, R.M.; Rideal, E.K. Permeation, diffusion and solution of gases in organic polymers. Trans. Faraday Soc. 1939, 35, 628–643. [Google Scholar] [CrossRef]
- Rutherford, S.W.; Do, D.D. Review of time lag permeation technique as a method for characterisation of porous media and membranes. Adsorption 1997, 3, 283–312. [Google Scholar] [CrossRef]
- Zhao, C.; Ribeiro, M.D.R.; de Pinho, M.N.; Subrahmanyam, V.S.; Gil, C.L.; de Lima, A.P. Structural characteristics and gas permeation properties of polynorbornenes with retained bicyclic structure. Polymer 2001, 42, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Kruczek, B.; Frisch, H.L.; Chapanian, R. Analytical solution for the effective time lag of a membrane in a permeate tube collector in which Knudsen flow regime exists. J. Membr. Sci. 2005, 256, 57–63. [Google Scholar] [CrossRef]
- Kruczek, B.; Shemshaki, F.; Lashkari, S.; Chapanian, R.; Frisch, H.L. Effect of a resistance-free tank on the resistance to gas transport in high vacuum tube. J. Membr. Sci. 2006, 280, 29–36. [Google Scholar] [CrossRef]
- Lashkari, S.; Kruczek, B.; Frisch, H.L. General solution for the time lag of a single-tank receiver in the Knudsen flow regime and its implications for the receiver’s configuration. J. Membr. Sci. 2006, 283, 88–101. [Google Scholar] [CrossRef]
- Lashkari, S.; Kruczek, B. Effect of resistance to gas accumulation in multi-tank receivers on membrane characterization by the time lag method. Analytical approach for optimization of the receiver. J. Membr. Sci. 2010, 360, 442–453. [Google Scholar] [CrossRef]
- Saini, V.K.; Pinto, M.; Pires, J. High Pressure Adsorption Studies of Ethane and Ethylene on Clay-Based Adsorbent Materials. Sep. Sci. Technol. 2010, 46, 137–146. [Google Scholar] [CrossRef]
- Dymond, J.H.; Smith, E.B. The Virial Coefficients of Pure Gases and Mixtures: A Critical Compilation; Clarendon Press: Oxford, UK; Oxford University Press: New York, NY, USA, 1980. [Google Scholar]
- Rasband, W. NIH Image, ImageJ, 2012. Available online: http://rsbweb.nih.gov/ij/ (accessed on 20 March 2012).
- Gray, D.N.N. Polymeric Membranes for Artificial Lungs. Polym. Mater. Artif. Organs 1984, 151–162. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Green, D.D.W.; Southard, D.M.Z. Perry’s Chemical Engineers’ Handbook, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]











| - | Membrane | Thickness, l (µm) |
|---|---|---|
| Group 1 | PU0 | 112 ± 6 |
| PU5 | 115 ± 4 | |
| PU10 | 107 ± 4 | |
| PU15 | 112 ± 1 | |
| Group 2 | PU5-5 | 107 ± 4 |
| PU5-10 | 121 ± 2 | |
| PU10-5 | 109 ± 4 | |
| PU19-10 | 121 ± 2 | |
| PU15-5 | 103 ± 5 | |
| PU15-10 | 110 ± 3 |
| - | - | P (Barrer) | |||||
|---|---|---|---|---|---|---|---|
| - | Membrane | N2 | O2 | CO2 | N2 | O2 | CO2 |
| Group 1 | PU0 | 0.0082 | 0.0218 | 0.203 | 9 ± 0.5 | 24 ± 1.3 | 227 ± 12.2 |
| PU5 and PU10 | 0.0071 | 0.0171 | 0.168 | 8 ± 0.3 | 20 ± 0.7 | 193 ± 6.7 | |
| PU15 | 0.0059 | 0.0149 | 0.154 | 7 ± 0.1 | 17 ± 0.1 | 172 ± 1.5 | |
| - | N2 | O2 | CO2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| - | tlag | D | S | tlag | D | S | tlag | D | S |
| - | (s) | (s) | (s) | ||||||
| PU0 | 17.3 | 1.4 ± 0.1 | 7.1 ± 0.2 | 10.1 | 2.1 ± 0.4 | 11.3 ± 1.7 | 11.4 | 1.7 ± 0.2 | 140.0 ± 12 |
| PU5 | 17.8 | 1.2 ± 0.1 | 6.1 ± 0.6 | 11.4 | 1.9 ± 0.2 | 10.6 ± 0.9 | 14.0 | 1.6 ± 0.2 | 123.2 ± 13 |
| PU10 | 18.3 | 1.2 ± 0.1 | 8.4 ± 1.0 | 10.1 | 1.6 ± 0.2 | 11.9 ± 2.1 | 13.5 | 1.4 ± 0.1 | 160.0 ± 9 |
| PU15 | 21.1 | 1.0 ± 0.0 | 6.7 ± 0.2 | 11.6 | 1.7 ± 0.1 | 9.6 ± 1.2 | 14.9 | 1.4 ± 0.1 | 119.2 ± 7 |
| Molecule | Kinetic Diameter (Å) | Boiling Point (°C) |
|---|---|---|
| CO2 | 3.30 | −78.5 |
| O2 | 3.46 | −183 |
| N2 | 3.64 | −196 |
| - | - | |||
|---|---|---|---|---|
| Membrane | N2 | O2 | CO2 | |
| Group 2 | PU5-5 | 0.0070 | 0.0196 | 0.2070 |
| PU5-10 | 0.0062 | 0.0158 | 0.1671 | |
| PU10-5 | 0.0067 | 0.0181 | 0.1840 | |
| PU10-10 | 0.0053 | 0.0146 | 0.1565 | |
| PU15-5 | 0.0060 | 0.0168 | 0.1855 | |
| PU15-10 | 0.0055 | 0.0153 | 0.1582 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eusébio, T.M.; Martins, A.R.; Pon, G.; Faria, M.; Morgado, P.; Pinto, M.L.; Filipe, E.J.M.; de Pinho, M.N. Sorption/Diffusion Contributions to the Gas Permeation Properties of Bi-Soft Segment Polyurethane/Polycaprolactone Membranes for Membrane Blood Oxygenators. Membranes 2020, 10, 8. https://doi.org/10.3390/membranes10010008
Eusébio TM, Martins AR, Pon G, Faria M, Morgado P, Pinto ML, Filipe EJM, de Pinho MN. Sorption/Diffusion Contributions to the Gas Permeation Properties of Bi-Soft Segment Polyurethane/Polycaprolactone Membranes for Membrane Blood Oxygenators. Membranes. 2020; 10(1):8. https://doi.org/10.3390/membranes10010008
Chicago/Turabian StyleEusébio, Tiago M., Ana Rita Martins, Gabriela Pon, Mónica Faria, Pedro Morgado, Moisés L. Pinto, Eduardo J. M. Filipe, and Maria Norberta de Pinho. 2020. "Sorption/Diffusion Contributions to the Gas Permeation Properties of Bi-Soft Segment Polyurethane/Polycaprolactone Membranes for Membrane Blood Oxygenators" Membranes 10, no. 1: 8. https://doi.org/10.3390/membranes10010008
APA StyleEusébio, T. M., Martins, A. R., Pon, G., Faria, M., Morgado, P., Pinto, M. L., Filipe, E. J. M., & de Pinho, M. N. (2020). Sorption/Diffusion Contributions to the Gas Permeation Properties of Bi-Soft Segment Polyurethane/Polycaprolactone Membranes for Membrane Blood Oxygenators. Membranes, 10(1), 8. https://doi.org/10.3390/membranes10010008