Silica Nanoparticles Reinforced Ionogel as Nonvolatile and Stretchable Conductors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.3.1. The size of SNPs
2.3.2. Transmittance
2.3.3. Mechanical Characterization
2.3.4. Electrical Characterization
2.3.5. Stability of Ionogels in Open Air
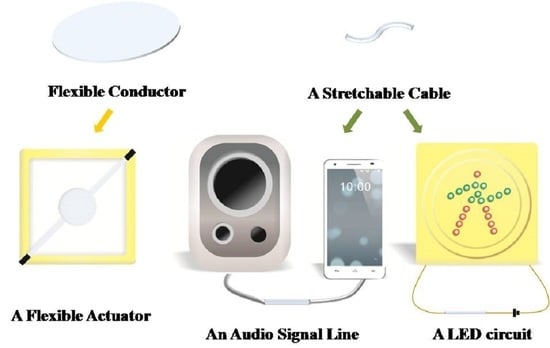
2.3.6. Demonstration of Potential Application
3. Results and Discussion
3.1. Mechanical Properties
3.2. Electrical Conductivity
3.3. Thermal Nonvolatility
3.4. Transparency Test
3.5. Electrodes for Flexible Actuators
3.6. Functional Testing of Stretchable Cables
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Madhvapathy, S.R.; Ma, Y.; Patel, M.; Krishnan, S.; Wei, C.; Li, Y.; Xu, S.; Feng, X.; Huang, Y.; Rogers, J.A. Epidermal electronic systems for measuring the thermal properties of human skin at depths of up to several millimeters. Adv. Funct. Mater. 2018, 28, 1802083. [Google Scholar] [CrossRef]
- Xu, B.; Akhtar, A.; Liu, Y.; Chen, H.; Yeo, W.-H.; Park, S.; Boyce, B.; Kim, H.; Yu, J.; Lai, H.-Y.; et al. Flexible Electronics: An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and Electrical Muscle Activation. Adv. Mater. 2016, 28, 4462–4471. [Google Scholar] [CrossRef]
- Sun, J.; Keplinger, C.; Whitesides, G.M.; Suo, Z.G. Ionic skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Miriyev, A.; Stack, K.; Lipson, H. Soft material for soft actuators. Nat. Commun. 2017, 8, 596. [Google Scholar] [CrossRef]
- Myny, K. The development of flexible integrated circuits based on thin-film transistors. Nat. Electron. 2018, 1, 30–39. [Google Scholar] [CrossRef]
- O’Doherty, J.E.; Lebedev, M.A.; Ifft, P.J.; Zhuang, K.Z.; Shokur, S.; Bleuler, H.; Nicolelis, M.A.L. Active tactile exploration using a brain–machine–brain interface. Nature 2011, 479, 228–231. [Google Scholar]
- Park, J.; Wang, S.; Li, M.; Ahn, C.; Hyun, J.K.; Kim, D.S.; Kim, D.K.; Rogers, J.A.; Huang, Y.; Jeon, S. Three-dimensional nanonetworks for giant stretchability in dielectrics and conductors. Nat. Commun. 2016, 3, 916. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Kaltenbrunner, M.; Yokota, T.; Jinno, H.; Kuribara, K.; Sekitani, T.; Someya, T. Printable elastic conductors with a high conductivity for electronic textile applications. Nat. Commun. 2015, 6, 7461. [Google Scholar] [CrossRef]
- Sahu, R.K.; Sudarshan, K.; Patra, K.; Bhaumik, S. Evaluation of area strain response of dielectric elastomer actuator using image processing technique. Electroact. Polym. Actuators Devices 2014, 905, c1–c90562. [Google Scholar]
- Fox, J.W.; Goulbourne, N.C. On the dynamic electromechanical loading of dielectric elastomer membranes. J. Mech. Phys. Solids 2008, 56, 2669–2686. [Google Scholar] [CrossRef]
- Chua, S.L.; Neo, X.H.; Lau, G.K. Multi-walled carbon nanotubes (MWCNT) as compliant electrodes for dielectric elastomer actuators. SPIE 2011, 16, S300–S305. [Google Scholar]
- Hwang, T.; Kwon, H.-Y.; Oh, J.-S.; Hong, J.-P.; Hong, S.C.; Lee, Y.; Choi, H.R.; Kim, K.J.; Bhuiya, M.H.; Nam, J.-D. Transparent actuator made with few layer graphene electrode and dielectric elastomer, for variable focus lens. Appl. Phys. Lett. 2013, 103, 023106. [Google Scholar] [CrossRef] [Green Version]
- Kaya, E.; Kakenov, N.; Altan, H.; Kocabas, C.; Esenturk, O. Multilayer Graphene Broadband Terahertz Modulators with Flexible Substrate. J. Infrared Millim. Terahertz Waves 2018, 39, 483–491. [Google Scholar] [CrossRef]
- Won, S.; Hwangbo, Y.; Lee, S.-K.; Kim, K.-S.; Kim, K.-S.; Lee, S.-M.; Lee, H.-J.; Ahn, J.-H.; Kim, J.-H.; Lee, S.-B. Double-layer CVD graphene as stretchable transparent electrodes. Nanoscale 2014, 6, 6057–6064. [Google Scholar] [CrossRef]
- Kim, T.-G.; Lee, J.-G.; Park, C.-W.; Hong, S.; Woo, W.; Hest, V.; Maikel, F.A.; Cho, D.-H.; Chung, Y.-D.; Yoon, S.S. Effect of supersonic spraying impact velocity on opto-electric properties of transparent conducting flexible films consisting of silver nanowire, ITO, and polyimide multilayers. J. Alloys Compd. 2018, 739, 653–659. [Google Scholar] [CrossRef]
- Datta, R.S.; Syed, N.; Zavabeti, A.; Jannat, A.; Daeneke, T. Flexible two-dimensional indium tin oxide fabricated using a liquid metal printing technique. Nat. Electron. 2020, 3, 51–58. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Yang, H.; Zhen, X.; Ma, L.; Williams, D.; Sun, X.; Lang, M. Highly transparent and flexible circuits through patterning silver nanowires into microfluidic channels. Chem. Commun. 2018, 54, 4923–4926. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, M.; Meng, L.; Wan, X.; Chen, Y. Flexible organic photovoltaics based on water-processed silver nanowire electrodes. Nat. Electron. 2019, 2, 513–520. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef]
- Cartmell, J.V.; Wolf, M.L. Medical Electrode. USA Patent US4617935 A, 21 October 1986. [Google Scholar]
- Rothemund, P.; Morelle, X.P.; Jia, K.; Whitesides, G.M.; Suo, Z. A Transparent membrane for active noise cancelation. Adv. Funct. Mater. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Ouyang, C.; Lu, T.; Li, F. Biofriendly, stretchable, and reusable hydrogel electronics as wearable force sensors. Small 2018, 14, 1801711. [Google Scholar] [CrossRef]
- Chen, B.; Lu, J.; Yang, C.; Yang, J.; Zhou, J.; Chen, Y.; Suo, Z. Highly stretchable and transparent ionogels as nonvolatile conductors for dielectric elastomer transducers. ACS Appl. Mater. Interfaces 2014, 6, 7840–7845. [Google Scholar] [CrossRef]
- Bideau, J.L.; Viau, J.; Vious, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Werner, S.; Haumann, M.; Wasserscheid, P. Ionic Liquids in Chemical Engineering. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 203–230. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.; He, Y.; Kim, B.S.; Lodge, T.P.; Frisbie, C.D. High-capacitance ion gel gate dielectrics with faster polarization response times for organic thin film transistors. Adv. Mater. 2008, 20, 686–690. [Google Scholar] [CrossRef]
- Bideau, J.L.; Ducros, J.B.; Soudan, P.; Guyomard, D. Solid-state electrode materials with ionic-liquid properties for energy storage: The lithium solid-state ionic-liquid concept. Adv. Funct. Mater. 2011, 21, 4073–4078. [Google Scholar] [CrossRef]
- Soavi, M.F. Ionic liquids for hybrid supercapacitors. Electrochem. Commun. 2004, 6, 566–570. [Google Scholar]
- Saricilar, S.; Antiohos, D.; Shu, K.; Whitten, P.G.; Wagner, K.; Wang, C.; Wallace, G.G. High strain stretchable solid electrolytes. Electrochem. Commun. 2013, 32, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Swapnil, S.N.; Kim, S.-J. Probing the energy conversion process in piezoelectric-driven electrochemical self-charging supercapacitor power cell using piezoelectrochemical spectroscopy. Nat. Commun. 2020, 11, 2351. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Gong, A.T.; Defnet, P.A.; Meabe, L.; Nelson, A. 3D printing ionogel auxetic frameworks for stretchable sensors. Adv. Mater. Technol. 2019, 4, 1900452. [Google Scholar] [CrossRef]
- Lodge, T.P. A unique platform for materials design. Science 2008, 321, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Susan, M.A.B.H.; Kaneko, T.; Noda, A.; Watanabe, M. Ion gels prepared by in situ radical polymerization of vinyl monomers in an ionic liquid and their characterization as polymer electrolytes. J. Amer. Chem. Soc. 2005, 127, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Guo, S.; Lu, Y.; Li, Y.; Lan, X.; Wu, D.; Li, R.; Wu, S.; Hu, X. Highly tough, Li-metal compatible organic-inorganic double-network solvate ionogel. Adv. Energy Mater. 2019, 9, 1900257. [Google Scholar] [CrossRef]
- Hyun, W.J.; Thomas, C.M.; Hersam, M.C. Nanocomposite ionogel electrolytes for solid-state rechargeable batteries. Adv. Energy Mater. 2020, 202002135. [Google Scholar] [CrossRef]
- Lai, F.; Yang, C.; Lian, R.; Chu, K.; Qin, J.; Zong, W.; Rao, D.; Hofkens, J.; Lu, X.; Liu, X. Three-phase boundary in cross-coupled micro-mesoporous networks enabling 3D-printed and ionogel-based Quasi-solid state micro-supercapacitors. Adv. Mater. 2020, 2002474. [Google Scholar] [CrossRef]
- Moganty, S.S.; Srivastava, S.; Lu, Y.; Schaefer, J.L.; Rizvi, S.A.; Archer, L.A. Ionic liquid-tethered nanoparticle suspensions: A novel class of ionogels. Chem. Mater. 2012, 24, 1386–1392. [Google Scholar] [CrossRef]
- Wu, F.; Chen, N.; Chen, R.; Wang, L.; Li, L. Organically modified silica-supported ionogels electrolyte for high temperature lithium-ion batteries. Nano Energy 2017, 31, 9–18. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Li, L.; Chen, R.; Guo, S. Ionogel electrolytes for high-performance lithium batteries: A review. Adv. Energy Mater. 2018, 8, 1702675. [Google Scholar] [CrossRef]
- Liu, J.; Nie, N.; Wang, H.; Chen, Z.; Ji, Z.; Duan, X.; Huang, Y. A zinc ion yarn battery with high capacity and fire retardancy based on a SiO2 nanoparticle doped ionogel electrolyte. Soft Matter 2020, 16, 7432–7437. [Google Scholar] [CrossRef] [PubMed]
- Stber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Ge, J.; Goebl, J.; Yin, Y. A Self-templated route to hollow silica microspheres. J. Phys. Chem. C 2009, 113, 3168–3175. [Google Scholar] [CrossRef]








| Sample | Electroconductivity (S/m) |
|---|---|
| blank | 1.28 ± 0.07 |
| SNP 0.5% | 1.23 ± 0.07 |
| SNP 1.0% | 1.09 ± 0.06 |
| SNP 2.0% | 1.11 ± 0.06 |
| SNP 3.0% | 1.10 ± 0.06 |
| SNP 4.0% | 1.12 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Li, Z.; Huang, P.; Lu, Y.; Wang, P. Silica Nanoparticles Reinforced Ionogel as Nonvolatile and Stretchable Conductors. Membranes 2020, 10, 354. https://doi.org/10.3390/membranes10110354
Zhang S, Li Z, Huang P, Lu Y, Wang P. Silica Nanoparticles Reinforced Ionogel as Nonvolatile and Stretchable Conductors. Membranes. 2020; 10(11):354. https://doi.org/10.3390/membranes10110354
Chicago/Turabian StyleZhang, Shanshan, Zhen Li, Pei Huang, Yamei Lu, and Pengfei Wang. 2020. "Silica Nanoparticles Reinforced Ionogel as Nonvolatile and Stretchable Conductors" Membranes 10, no. 11: 354. https://doi.org/10.3390/membranes10110354
APA StyleZhang, S., Li, Z., Huang, P., Lu, Y., & Wang, P. (2020). Silica Nanoparticles Reinforced Ionogel as Nonvolatile and Stretchable Conductors. Membranes, 10(11), 354. https://doi.org/10.3390/membranes10110354