Influence of Sodium Hypochlorite Treatment on Pore Size Distribution of Polysulfone/Polyvinylpyrrolidone Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Spinning and Characterization
2.3. Measurement of Pore Size and Distribution
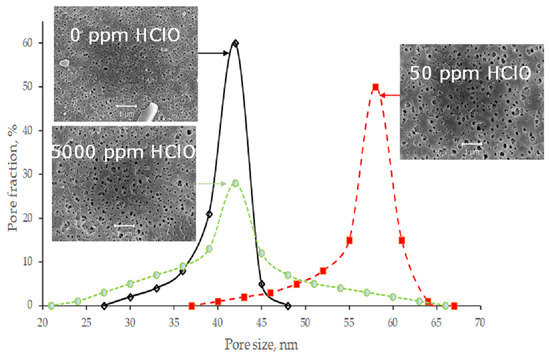
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tweddle, T.A.; Kutowy, O.; Thayer, W.L.; Sourirajan, S. Polysulfone ultrafiltration membranes. Ind. Eng. Chem. Res. 1983, 22, 320–326. [Google Scholar] [CrossRef]
- Feng, X.; Ivory, J.; Rajan, V.S.V. Air separation by integrally asymmetric hollow-fiber membranes. AIChE J. 1999, 45, 2142–2152. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Aroon, M.A.; Ismail, A.F.; Montazer-Rahmati, M.M.; Matsuura, T. Morphology and permeation properties of polysulfone membranes for gas separation: Effects of non-solvent additives and co-solvent. Sep. Purif. Technol. 2010, 72, 194–202. [Google Scholar] [CrossRef]
- Tsai, H.A.; Kuo, C.Y.; Lin, J.H.; Wang, D.M.; Deratani, A.; Pochat-Bohatier, C.; Lee, K.R.; Lai, J.Y. Morphology control of polysulfone hollow fiber membranes via water vapor induced phase separation. J. Membr. Sci. 2006, 278, 390–400. [Google Scholar] [CrossRef]
- Wang, D.; Teo, W.K.; Li, K. Preparation and characterization of high-flux polysulfone hollow fibre gas separation membranes. J. Membr. Sci. 2002, 204, 247–256. [Google Scholar] [CrossRef]
- Dibrov, G.; Ivanov, M.; Semyashkin, M.; Sudin, V.; Fateev, N.; Kagramanov, G. Elaboration of High Permeable Macrovoid Free Polysulfone Hollow Fiber Membranes for Air Separation. Fibers 2019, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Apel, P.Y.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of Membrane Science Development. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Matveev, D.N.; Vasilevskii, V.P.; Borisov, I.L.; Volkov, V.V.; Volkov, A.V. Effects of dry-jet wet spinning parameters on properties of polysulfone hollow fiber membranes. Russ. J. Appl. Chem. 2020, 93, 554–563. [Google Scholar] [CrossRef]
- Volkov, V.V.; Bildukevich, A.V.; Dibrov, G.A.; Usoskiy, V.V.; Kasperchik, V.P.; Vasilevsky, V.P.; Novitsky, E.G. Elaboration of composite hollow fiber membranes with selective layer from poly[1-(trimethylsylil)1-propyne] for regeneration of aqueous alkanolamine solutions. Pet. Chem. 2013, 53, 619–626. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Hliavitskaya, T.A.; Penkova, A.V.; Ermakov, S.S.; Ulbricht, M. Modification of Polysulfone Ultrafiltration Membranes via Addition of Anionic Polyelectrolyte Based on Acrylamide and Sodium Acrylate to the Coagulation Bath to Improve Antifouling Performance in Water Treatment. Membranes 2020, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wu, M.; Van der Bruggen, B.; Lei, L.; Zhu, L. Effect of TiO2 content on the properties of polysulfone nanofiltration membranes modified with a layer of TiO2–graphene oxide. Sep. Purif. Technol. 2020, 242, 116770. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Ermakov, S.S.; Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Hliavitskaya, T.A.; Ulbricht, M. One-Step Preparation of Antifouling Polysulfone Ultrafiltration Membranes via Modification by a Cationic Polyelectrolyte Based on Polyacrylamide. Polymers 2020, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Luo, J.; Wei, J.; Woodley, J.; Daugaard, A.E.; Pinelo, M. Commercial polysulfone membranes pretreated with ethanol and NaOH: Effects on permeability, selectivity and antifouling properties. Sep. Purif. Technol. 2019, 219, 82–89. [Google Scholar] [CrossRef]
- Li, X.; Janke, A.; Formanek, P.; Fery, A.; Stamm, M.; Tripathi, B.P. High permeation and antifouling polysulfone ultrafiltration membranes with in situ synthesized silica nanoparticles. Mater. Today Commun. 2020, 22, 100784. [Google Scholar] [CrossRef]
- Dong, X.; Jeong, T.J.; Kline, E.; Banks, L.; Grulke, E.; Harris, T.; Escobar, I.C. Eco-friendly solvents and their mixture for the fabrication of polysulfone ultrafiltration membranes: An investigation of doctor blade and slot die casting methods. J. Membr. Sci. 2020, 614, 118510. [Google Scholar] [CrossRef]
- Kostyanaya, M.; Bazhenov, S.; Borisov, I.; Plisko, T.; Vasilevsky, V. Surface Modified Polysulfone Hollow Fiber Membranes for Ethane/Ethylene Separation Using Gas-Liquid Membrane Contactors with Ionic Liquid-Based Absorbent. Fibers 2019, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Penkova, A.V.; Dmitrenko, M.E.; Sokolova, M.P.; Chen, B.; Plisko, T.V.; Markelov, D.A.; Ermakov, S.S. Impact of fullerene loading on the structure and transport properties of polysulfone mixed-matrix membranes. J. Mater. Sci. 2016, 51, 7652–7659. [Google Scholar] [CrossRef]
- Malakhov, A.O.; Anokhina, T.S.; Petrova, D.A.; Vinokurov, V.A.; Volkov, A.V. Nanocellulose as a Component of Ultrafiltration Membranes. Pet. Chem. 2018, 58, 923–933. [Google Scholar] [CrossRef]
- Rouaix, S.; Causserand, C.; Aimar, P. Experimental study of the effects of hypochlorite on polysulfone membrane properties. J. Membr. Sci. 2006, 277, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Astakhov, E.Y.; Kolganov, I.M.; Klinshpont, E.R.; Tsarin, P.G.; Kalacheva, A.A. Influence of polyvinylpyrrolidone on morphology, hydrophilicity, and performance of polyethersulfone microfiltration membranes. Pet. Chem. 2012, 52, 557–564. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and membrane hydrophilic modification to reduce membrane fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Bildyukevich, A.V.; Plisko, T.V.; Liubimova, A.S.; Volkov, V.V.; Usosky, V.V. Hydrophilization of polysulfone hollow fiber membranes via addition of polyvinylpyrrolidone to the bore fluid. J. Membr. Sci. 2017, 524, 537–549. [Google Scholar] [CrossRef]
- Yi, Z.; Zhu, L.P.; Xu, Y.Y.; Zhao, Y.F.; Ma, X.T.; Zhu, B.K. Polysulfone-based amphiphilic polymer for hydrophilicity and fouling-resistant modification of polyethersulfone membranes. J. Membr. Sci. 2010, 365, 25–33. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Pang, W.Y.; Shafie, Z.M.H.M.; Zaulkiflee, N.D. PES/PVP/TiO2 mixed matrix hollow fiber membrane with antifouling properties for humic acid removal. J. Water Proc. Eng. 2019, 31, 100827. [Google Scholar] [CrossRef]
- Jaleh, B.; Zare, E.; Azizian, S.; Qanati, O.; Nasrollahzadeh, M.; Varma, R.S. Preparation and Characterization of Polyvinylpyrrolidone/Polysulfone Ultrafiltration Membrane Modified by Graphene Oxide and Titanium Dioxide for Enhancing Hydrophilicity and Antifouling Properties. J. Inorg. Organomet. Polym. 2020, 30, 2213–2223. [Google Scholar] [CrossRef]
- Causserand, C.; Pellegrin, B.; Rouch, J.C. Effects of sodium hypochlorite exposure mode on PES/PVP ultrafiltration membrane degradation. Water Res. 2015, 85, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Wienk, I.M.; Meuleman, E.E.B.; Borneman, Z.; Van Den Boomgaard, T.; Smolders, C.A. Chemical treatment of membranes of a polymer blend: Mechanism of the reaction of hypochlorite with poly (vinyl pyrrolidone). J. Polym. Sci. A Polym. Chem. 1995, 33, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Xie, P.; de Lannoy, C.F.; Ma, J.; Wiesner, M.R. Chlorination of polyvinyl pyrrolidone–polysulfone membranes: Organic compound release, byproduct formation, and changes in membrane properties. J. Membr. Sci. 2015, 489, 28–35. [Google Scholar] [CrossRef]
- Qin, J.J.; Wong, F.S.; Li, Y.; Liu, Y.T. A high flux ultrafiltration membrane spun from PSU/PVP (K90)/DMF/1,2-propandiol. J. Membr. Sci. 2003, 211, 139. [Google Scholar] [CrossRef]
- Wolff, S.H.; Zydney, A.L. Effect of bleach on the transport characteristics of polysulfone hemodialyzers. J. Membr. Sci. 2004, 243, 389–399. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Gao, F.; Tao, H.; Chen, Y.; Zhang, H. Impact of sodium hypochlorite (NaClO) on polysulfone (PSF) ultrafiltration membranes: The evolution of membrane performance and fouling behavior. Sep. Purif. Technol. 2017, 175, 238–247. [Google Scholar] [CrossRef]
- Pellegrin, B.; Mezzari, F.; Hanafi, Y.; Szymczyk, A.; Remigy, J.-C.; Causserand, C. Filtration performance and pore size distribution of hypochlorite aged PES/PVP ultrafiltration membranes. J. Membr. Sci. 2015, 474, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, S.; Su, Q.; Wen, G.; Huang, T. Effects of Hydrogen Peroxide and Sodium Hypochlorite Aging on Properties and Performance of Polyethersulfone Ultrafiltration Membrane. Int. J. Environ. Res. Public Health 2019, 16, 3972. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, Y.; Szymczyk, A.; Rabiller-Baudry, M.; Baddari, K. Degradation of poly (ether sulfone)/polyvinylpyrrolidone membranes by sodium hypochlorite: Insight from advanced electrokinetic characterizations. Environ. Sci. Technol. 2014, 48, 13419–13426. [Google Scholar] [CrossRef]
- Chokki, J.; Darracq, G.; Pölt, P.; Baron, J.; Gallard, H.; Joyeux, M.; Teychené, B. Investigation of Poly (ethersulfone)/Polyvinylpyrrolidone ultrafiltration membrane degradation by contact with sodium hypochlorite through FTIR mapping and two-dimensional correlation spectroscopy. Polym. Degrad. Stab. 2019, 161, 131–138. [Google Scholar] [CrossRef]
- Ravereau, J.; Fabre, A.; Brehant, A.; Bonnard, R.; Sollogoub, C.; Verdu, J. Ageing of polyvinylidene fluoride hollow fiber membranes in sodium hypochlorite solutions. J. Membr. Sci. 2016, 505, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Mavukkandy, M.O.; Bilad, M.R.; Giwa, A.; Hasan, S.W.; Arafat, H.A. Leaching of PVP from PVDF/PVP blend membranes: Impacts on membrane structure and fouling in membrane bioreactors. J. Mater. Sci. 2016, 51, 4328–4341. [Google Scholar] [CrossRef]
- Qin, J.J.; Li, Y.; Lee, L.S.; Lee, H. Cellulose acetate hollow fiber ultrafiltration membranes made from CA/PVP 360 K/NMP/water. J. Membr. Sci. 2003, 218, 173–183. [Google Scholar] [CrossRef]
- Qin, J.J.; Cao, Y.M.; Li, Y.Q.; Li, Y.; Oo, M.H.; Lee, H. Hollow fiber ultrafiltration membranes made from blends of PAN and PVP. Sep. Purif. Technol. 2004, 36, 149–155. [Google Scholar] [CrossRef]
- Ivanov, M.V.; Dibrov, G.A.; Loyko, A.V.; Varezhkin, A.V.; Kagramanov, G.G. Techniques to manage geometry characteristics of hollow-fiber membranes. Theor. Found. Chem. Eng. 2016, 50, 316–324. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Usosky, V.V.; Volkov, V.V. Influence of the concentration and molecular weight of polyethylene glycol on the structure and permeability of polysulfone hollow fiber membranes. Pet. Chem. 2016, 56, 321–329. [Google Scholar] [CrossRef]
- Dibrov, G.; Ivanov, M.; Semyashkin, M.; Sudin, V.; Kagramanov, G. High-pressure aging of asymmetric Torlon® hollow fibers for helium separation from natural gas. Fibers 2018, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Zeman, L.J.; Zydney, A. Microfiltration and Ultrafiltration: Principles and Applications; Marcel Dekker: New York, NY, USA, 1996; pp. 197–201. ISBN 9781351431514. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, V. Impact of chemical cleaning on properties and functioning of polyethersulfone membranes. J. Membr. Sci. 2007, 305, 176–184. [Google Scholar] [CrossRef]
- Bormashenko, E.; Pogreb, R.; Whyman, G.; Bormashenko, Y.; Jager, R.; Stein, T.; Schechter, A.; Aurbach, D. The reversible giant change in the contact angle on the polysulfone and polyethersulfone films exposed to UV irradiation. Langmuir 2008, 24, 5977–5980. [Google Scholar] [CrossRef]



| C, ppm | 0 | 25 | 50 | 250 | 500 | 2500 | 5000 |
|---|---|---|---|---|---|---|---|
| P/l, L/(m2·h·bar) | 270 ± 30 | 1230 ± 90 | 1400 ± 150 | 1210 ± 150 | 810 ± 110 | 530 ± 70 | 500 ± 120 |
| pH | 6.8 | 7.0 | 7.2 | 9.3 | 10.2 | 11.2 | 11.5 |
| C, ppm | 0 | 50 | 5000 |
|---|---|---|---|
| θ(H2O), deg. | 50 ± 6 | 60 ± 5 | 90 ± 5 |
| θ(10% BuOH), deg. | 30 ± 1 | 30 ± 1 | 30 ± 1 |
| Pburst(H2O), bar | >10 | >10 | >10 |
| Pburst (BuOH/H2O), bar | 4.17 | 2.34 | 4.03 |
| Mean pore size (LLDP), nm | 42 | 58 | 42 |
| Mean inner pore size (SEM), nm | 54 | 62 | 56 |
| Mean outer pore size (SEM), nm | 93 | 152 | 150 |
| Maximum pore size (LLDP), nm | 48 | 67 | 66 |
| Maximum inner pore size (SEM), nm | 120 | 185 | 126 |
| Maximum outer pore size (SEM), nm | 120 | 193 | 190 |
| Porosity (LLDP), % | 2.7 | 7.4 | 5.1 |
| Inner surface porosity (SEM), % | 6.8 | 12.9 | 9.3 |
| Outer surface porosity (SEM), % | 16.5 | 43.2 | 41.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibrov, G.; Kagramanov, G.; Sudin, V.; Grushevenko, E.; Yushkin, A.; Volkov, A. Influence of Sodium Hypochlorite Treatment on Pore Size Distribution of Polysulfone/Polyvinylpyrrolidone Membranes. Membranes 2020, 10, 356. https://doi.org/10.3390/membranes10110356
Dibrov G, Kagramanov G, Sudin V, Grushevenko E, Yushkin A, Volkov A. Influence of Sodium Hypochlorite Treatment on Pore Size Distribution of Polysulfone/Polyvinylpyrrolidone Membranes. Membranes. 2020; 10(11):356. https://doi.org/10.3390/membranes10110356
Chicago/Turabian StyleDibrov, George, George Kagramanov, Vladislav Sudin, Evgenia Grushevenko, Alexey Yushkin, and Alexey Volkov. 2020. "Influence of Sodium Hypochlorite Treatment on Pore Size Distribution of Polysulfone/Polyvinylpyrrolidone Membranes" Membranes 10, no. 11: 356. https://doi.org/10.3390/membranes10110356
APA StyleDibrov, G., Kagramanov, G., Sudin, V., Grushevenko, E., Yushkin, A., & Volkov, A. (2020). Influence of Sodium Hypochlorite Treatment on Pore Size Distribution of Polysulfone/Polyvinylpyrrolidone Membranes. Membranes, 10(11), 356. https://doi.org/10.3390/membranes10110356