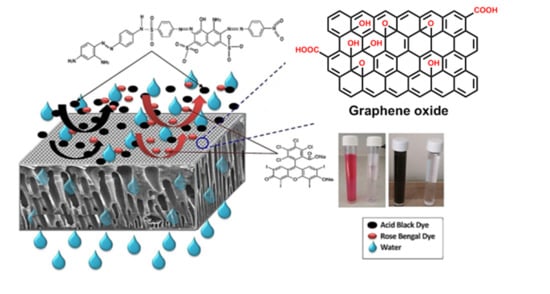
Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes
Abstract
:1. Introduction
2. Experimental Work
2.1. Chemicals
2.2. Preparation of the Graphene Oxide (GO)
2.3. Preparation of the Membrane
2.4. Membrane Characteristics
2.4.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Atomic Force Microscopy (AFM)
2.4.4. Contact Angle
2.4.5. Porosity
2.4.6. Pore Radius
2.4.7. Mechanical Properties (Tensile Strength)
2.4.8. Zeta Potential Test
2.4.9. Membrane Filtration Experiments
3. Results and Discussion
3.1. Characterization of the Composite Membranes
3.1.1. FTIR-ATR Spectrum Results
3.1.2. Morphology Examination by Scanning Electron Microscopy (SEM)
3.1.3. Atomic Force Microscopy (AFM) and Mean Pore Radius
3.1.4. Thickness and Porosity of Pristine PES and PES–PVP–GO Membranes
3.1.5. Hydrophilicity of the PES and GO/PES Composite Membranes
3.1.6. Mechanical Properties (Tensile Strength)
3.2. Performance of Various PES Membranes
3.2.1. Permeation Flux and Dyes Removal
3.2.2. Integrity and Stability of the PES Membranes during Long-Term Performance
3.2.3. Flux Recovery Efficiency
3.2.4. Effect of the Feed pH on the Permeation Flux and Dye Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Petersen, L.; Heynen, M.; Pellicciotti, F. Freshwater Resources: Past, Present, Future. Int. Encycl. Geogr. 2017, 1–11. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Baltaru, M.C.; Tang, Y.P.; Bernstein, N.J.; Gao, P.; Balta, S.; Vlad, M.; Volodin, A.; Sotto, A.; et al. Tight ultrafiltration membranes for enhanced separation of dyes and Na2SO4 during textile wastewater treatment. J. Membr. Sci. 2016, 514, 217–228. [Google Scholar] [CrossRef]
- Van Der Bruggen, B. Mechanisms of retention and flux decline for the nanofiltration of dye baths from the textile industry. Sep. Purif. Technol. 2001, 22, 519–528. [Google Scholar] [CrossRef]
- Koyuncu, I. Reactive dye removal in dye/salt mixtures by nanofiltration fibers containing vinylsulphone dyes: Effects of feed concentration and cross flow velocity. Desalination 2002, 143, 243–253. [Google Scholar] [CrossRef]
- Majewska-Nowak, K. Ultrafiltration of dye solutions in the presence of cationic and anionic surfactants. Environ. Prot. Eng. 2009, 35, 111–121. [Google Scholar]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Alsalhy, Q.F.; Ibrahim, S.S.; Khaleel, S.R. Performance of vacuum poly(propylene) membrane distillation (VMD) for saline water desalination. Chem. Eng. Process. Process. Intensif. 2017, 120, 68–80. [Google Scholar] [CrossRef]
- Al-Ani, D.M.; Al-Ani, F.H.; Alsalhy, Q.F.; Ibrahim, S.S. Preparation and characterization of ultrafiltration membranes from PPSU-PES polymer blend for dye removal. Chem. Eng. Commun. 2019, 1–19. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Rashid, K.T.; Ibrahim, S.S.; Ghanim, A.H.; Bruggen, B.V.; Luis, P.; Zablouk, M. Poly(vinylidene fluoride-co-hexafluropropylene) (PVDF-co-HFP) hollow fiber membranes prepared from PVDF-co-HFP/PEG-600Mw/DMAC solution for membrane distillation. J. Appl. Polym. Sci. 2013, 129, 3304–3313. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Al-Ani, F.H.; Al-Najar, A.E.; Jabuk, S.I. A study of the effect of embedding ZnO-NPs on PVC membrane performance use in actual hospital wastewater treatment by membrane bioreactor. Chem. Eng. Process. Process. Intensif. 2018, 130, 262–274. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Al-Ani, F.H.; Al-Najar, A.E. A new Sponge-GAC-Sponge membrane module for submerged membrane bioreactor use in hospital wastewater treatment. Biochem. Eng. J. 2018, 133, 130–139. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Aljumaily, M.A.A.M.M.; Alsaadi, M.A.; Hashim, N.A.; Alsalhy, Q.F.; Das, R.; Mjalli, F.S. Embedded high-hydrophobic CNMs prepared by CVD technique with PVDF-co-HFP membrane for application in water desalination by DCMD. Desalin. Water Treat. 2019, 142, 37–48. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Hashim, N.A.; Alsalhy, Q.F.; Mjalli, F.S.; Atieh, M.A.; Al-Harrasi, A. PVDF-co-HFP/superhydrophobic acetylene-based nanocarbon hybrid membrane for seawater desalination via DCMD. Chem. Eng. Res. Des. 2018, 138, 248–259. [Google Scholar] [CrossRef]
- Jamed, M.J.; AlAnezi, A.A.; Alsalhy, Q.F. Effects of embedding functionalized multi-walled carbon nanotubes and alumina on the direct contact poly(vinylidene fluoride-co-hexafluoropropylene) membrane distillation performance. Chem. Eng. Commun. 2018, 206, 1035–1057. [Google Scholar] [CrossRef]
- Sadiq, A.J.; Shabeeb, K.M.; Khalil, B.I.; Alsalhy, Q.F. Effect of embedding MWCNT-g-GO with PVC on the performance of PVC membranes for oily wastewater treatment. Chem. Eng. Commun. 2019, 207, 1–18. [Google Scholar] [CrossRef]
- Al-Ani, F.H.; Alsalhy, Q.F.; Raheem, R.S.; Rashid, K.T.; Figoli, A. Experimental investigation of the effect of implanting tio2-nps on pvc for long-term uf membrane performance to treat refinery wastewater. Membranes 2020, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Hussein, S.S.; Ibrahim, S.S.; Toma, M.A.; Alsalhy, Q.F.; Drioli, E. Novel chemical modification of polyvinyl chloride membrane by free radical graft copolymerization for direct contact membrane distillation (DCMD) application. J. Membr. Sci. 2020, 611, 118266. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kaner, R.B. Materials Science: Graphene-Based Materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, L.; Bai, H.; Hong, W.; Li, C.; Shi, G. Chemically Converted Graphene Induced Molecular Flattening of 5,10,15,20-Tetrakis(1-methyl-4-pyridinio)porphyrin and Its Application for Optical Detection of Cadmium(II) Ions. J. Am. Chem. Soc. 2009, 131, 13490–13497. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Lv, W.; Zhang, C.; Li, Z.; Su, R.; Qi, W.; Yang, Q.-H.; He, Z. High-performance ultrafiltration membranes based on polyethersulfone–graphene oxide composites. RSC Adv. 2013, 3, 21394–21397. [Google Scholar] [CrossRef]
- Sabri, A.; Marino, T.; Parsa, J.B.; Galiano, F.; Ursino, C.; Garcìa, H.; Puche, M.; Figoli, A. Polyvinylidene Fluoride-Graphene Oxide Membranes for Dye Removal under Visible Light Irradiation. Polymers 2020, 12, 1509. [Google Scholar] [CrossRef]
- Junaidi, N.F.; Khalil, N.A.; Jahari, A.F.; Shaari, N.Z.; Shahruddin, M.Z.; Alias, N.H.; Othman, N.H. Effect of Graphene Oxide (GO) on the Surface Morphology & Hydrophilicity of Polyethersulfone (PES). Mater. Sci. Eng. 2018, 358, 012047. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mansoor, B.; Mansour, A.; Khraisheh, M. Functional graphene nanosheets: The next generation membranes for water desalination. Desalination 2015, 356, 208–225. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Makhetha, T.; Moutloali, R. Antifouling properties of Cu(tpa)@GO/PES composite membranes and selective dye rejection. J. Membr. Sci. 2018, 554, 195–210. [Google Scholar] [CrossRef]
- Gholami, N.; Mahdavi, H. Nanofiltration composite membranes of polyethersulfone and graphene oxide and sulfonated graphene oxide. Adv. Polym. Technol. 2018, 37, 3529–3541. [Google Scholar] [CrossRef] [Green Version]
- Abdelkarim, A.A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Feng, M.; Liu, Y.; Deng, H.; Lu, J. Fabrication of graphene oxide blended polyethersulfone membranes via phase inversion assisted by electric field for improved separation and antifouling performance. J. Membr. Sci. 2019, 577, 41–50. [Google Scholar] [CrossRef]
- Luque-Alled, J.M.; Abdel-Karim, A.; Alberto, M.; Leaper, S.; Perez-Page, M.; Huang, K.; Vijayaraghavan, A.; El-Kalliny, A.S.; Holmes, S.M.; Gorgojo, P. Polyethersulfone membranes: From ultrafiltration to nanofiltration via the incorporation of APTS functionalized-graphene oxide. Sep. Purif. Technol. 2020, 230, 230. [Google Scholar] [CrossRef]
- Kumar, M.; McGlade, D.; Ulbricht, M.; Lawler, J. Quaternized polysulfone and graphene oxide nanosheet derived low fouling novel positively charged hybrid ultrafiltration membranes for protein separation. RSC Adv. 2015, 5, 51208–51219. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.; Shariaty-Niassar, M.; Matsuura, T.; Ismail, A.F. Janus graphene oxide nanosheet: A promising additive for enhancement of polymeric membranes performance prepared via phase inversion. J. Colloid Interface Sci. 2018, 527, 10–24. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Shan, M.; Li, Y.; Li, B.; Niu, J.; Zhou, B.; Qian, X. Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2014, 458, 1–13. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, X.; Chen, J.; Wang, G.; Yang, F. Highly effective antifouling performance of PVDF/graphene oxide composite membrane in membrane bioreactor (MBR) system. Desalination 2014, 340, 59–66. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Tyler, J.L.; Stretz, H.A.; Wells, M.J. Effects of a dual nanofiller, nano-TiO2 and MWCNT, for polysulfone-based nanocomposite membranes for water purification. Desalination 2015, 372, 47–56. [Google Scholar] [CrossRef]
- Galiano, F.; Ghanim, A.H.; Rashid, K.T.; Marino, T.; Simone, S.; Alsalhy, Q.F.; Figoli, A. Preparation and characterization of green polylactic acid (PLA) membranes for organic/organic separation by pervaporation. Clean Technol. Environ. Policy 2019, 21, 109–120. [Google Scholar] [CrossRef]
- Ismael, A.E. Nano Submerged Membrane Bioreactor for Hospital Wastewater Treatment; University of Technology: Baghdad, Iraq, 2017. [Google Scholar]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H.D.A. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Ghezelbash, M.; Shabanian, M.; Aryanasab, F.; Saeb, M.R. Efficient removal of cationic dyes from colored wastewaters by dithiocarbamate-functionalized graphene oxide nanosheets: From synthesis to detailed kinetics studies. J. Taiwan Inst. Chem. Eng. 2017, 81, 239–246. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Sun, M.; Su, Y.; Mu, C.; Jiang, Z. Improved Antifouling Property of PES Ultrafiltration Membranes Using Additive of Silica−PVP Nanocomposite. Ind. Eng. Chem. Res. 2010, 49, 790–796. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Novel antibifouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes. Sep. Purif. Technol. 2012, 90, 69–82. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Mousavi, S.A.; Rashidi, A.; Jafari, A.; Nazmara, S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J. Environ. Heal. Sci. Eng. 2015, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.; Pan, X.; Zhang, L.; Chen, H.; Gao, C. Preparation and properties of functionalized carbon nanotube/PSF blend ultrafiltration membranes. J. Membr. Sci. 2009, 342, 165–172. [Google Scholar] [CrossRef]
- Lohokare, H.; Muthu, M.; Agarwal, G.; Kharul, U.K. Effective arsenic removal using polyacrylonitrile-based ultrafiltration (UF) membrane. J. Membr. Sci. 2008, 320, 159–166. [Google Scholar] [CrossRef]
- Xia, S.; Yao, L.; Zhao, Y.; Li, N.; Zheng, Y. Preparation of graphene oxide modified polyamide thin film composite membranes with improved hydrophilicity for natural organic matter removal. Chem. Eng. J. 2015, 280, 720–727. [Google Scholar] [CrossRef]
- Thomas, T.E.; Al Aani, S.; Oatley-Radcliffe, D.L.; Williams, P.M.; Hilal, N. Laser Doppler Electrophoresis and electro-osmotic flow mapping: A novel methodology for the determination of membrane surface zeta potential. J. Membr. Sci. 2017, 523, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Boukoussa, B.; Hamacha, R.; Morsli, A.; Bengueddach, A. Adsorption of yellow dye on calcined or uncalcined Al-MCM-41 mesoporous materials. Arab. J. Chem. 2017, 10, S2160–S2169. [Google Scholar] [CrossRef] [Green Version]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Lau, W.; Ismail, A.F. Application of response surface methodology in pes/speek blend NF membrane for dyeing solution treatment. Membr. Water Treat. 2010, 1, 49–60. [Google Scholar] [CrossRef]














| Membrane Label | PES Content (wt.%) | PVP Content (wt.%) | DMSO Content (wt.%) | GO Content (wt.%) |
|---|---|---|---|---|
| 1 | 21 | 1 | 78 | 0 |
| 2 | 21 | 1 | 78 | 0.1 |
| 3 | 21 | 1 | 78 | 0.2 |
| 4 | 21 | 1 | 78 | 0.3 |
| 5 | 21 | 1 | 78 | 0.5 |
| 6 | 21 | 1 | 78 | 1.5 |
| 7 | 21 | 1 | 78 | 2 |
| Percent of GO in PES Membrane | Top Surface Mean Pore Radius (nm) | Mean Roughness (nm) |
|---|---|---|
| 0 | 8.25 | 45.5 |
| 0.1 | 5.29 | 36 |
| 0.2 | 11.55 | 111 |
| 0.3 | 14.06 | 77.1 |
| 0.5 | 14.59 | 22 |
| 1.5 | 15.59 | 41 |
| 2 | 15.22 | 28.9 |
| Polymer (wt.%) | Fillers (wt.%) | Pore-Formers PVP (wt.%) | Permeate (LMH bar) | Rejection (%) | Ref. |
|---|---|---|---|---|---|
| PES (16) | * Cu(TPA)@GO | PVP (2) | 24–75 | 15% Methyl blue 65% Methyl orange 90% Congo red | [32] |
| PES (18) | sulfonated-GO (1) | PVP (1) | 9.1 | 83.9% Acid blue 83.5% Bismark Brown | [33] |
| PES (20) | GO (0.3) | T904 (5) | 245 | 62.3% sunset yellow (SY) dye 35.4% acridine orange (AO) dye | [34] |
| PES (18) | GO (0.008) | PVP (1) | 289.86 | 86.58% methyl red | [35] |
| PES (16) | * APTS-GO (0.1) | PVP-40 (1) | 9.9 | 97.4% SY 96.5% AO 51.6% MgSO4 | [36] |
| PSf (16) | GO (0–5) | No additive used | 87.5–150 | No rejection data | [37] |
| PSf (15) | Janus GO (0.1–1) | No additive used | 11.5–115 | 92% BSA | [38] |
| PVDF (15) | GO (0–2) | PVP (1) | 188.36 | 52% BSA | [39] |
| PVDF (12) | GO (0.5, 1 and 2%) | PVP (5) | 552.92 | 87.11% COD 93.75% NH4-N | [40] |
| PES (21) | GO (0.1–2) wt.% | PVP (1) | 116.5 | 99.7% Acid black (AB) dye 99% Rose Bengal dye (RB) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadhim, R.J.; Al-Ani, F.H.; Al-shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes 2020, 10, 366. https://doi.org/10.3390/membranes10120366
Kadhim RJ, Al-Ani FH, Al-shaeli M, Alsalhy QF, Figoli A. Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes. 2020; 10(12):366. https://doi.org/10.3390/membranes10120366
Chicago/Turabian StyleKadhim, Rana J., Faris H. Al-Ani, Muayad Al-shaeli, Qusay F. Alsalhy, and Alberto Figoli. 2020. "Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes" Membranes 10, no. 12: 366. https://doi.org/10.3390/membranes10120366
APA StyleKadhim, R. J., Al-Ani, F. H., Al-shaeli, M., Alsalhy, Q. F., & Figoli, A. (2020). Removal of Dyes Using Graphene Oxide (GO) Mixed Matrix Membranes. Membranes, 10(12), 366. https://doi.org/10.3390/membranes10120366