Treatment of Aqueous Effluents from Steel Manufacturing with High Thiocyanate Concentration by Reverse Osmosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Equipment and Procedure
- J: Overall permeate flux (L/m2 h).
- JS: Solute flux, Js=J·cP, (g/m2 h).
- k: Mass transfer coefficient (L/m2 h).
- cm: Solute concentration on the membrane surface (g/L).
- cB: Bulk solute concentration in the retentate (g/L).
- cP: Solute concentration in the permeate (g/L).
- A: Solvent (water) membrane permeability (L/m2 h bar).
- B: Solute membrane permeability (L/m2 h).
2.2. Analytical Methods
- pH and conductivity were determined by a potentiometric method, using a Mettler Toledo Seven Multi Dual pH (Columbus, OH, USA) and conductivity meter.
- Chemical oxygen demand (COD) was determined by refluxing a sample in strongly acid solution with a known excess of potassium dichromate and then measuring the absorbance of the mixture at a wavelength of 620 nm with a HACH DR/2010 spectrophotometer (HACH Co, Loveland, CO, USA) [24].
- Phenolic compounds were characterized by their absorbance at 280 nm [25], with a T80 ultraviolet/visible (UV/VIS) spectrophotometer (PG Instruments Ltd., Leicestershire, UK)
- Total cyanide, free cyanide and weak acid dissociable cyanide concentrations were determined with the same T80 UV/VIS spectrophotometer at 300 nm after the reaction of HCN with chloramine-T, and the addition of a pyridine-barbituric acid agent. The hydrogen cyanide is generated by the alkaline distillation [24] or of ultraviolet radiation [26] on the sample.
- The thiocyanate ion was analyzed by their reaction with Fe(NO3)3·9H2O at low pH, because it forms an intense red color, suitable for colorimetric determination by a PG Instruments Ltd. T80 UV/VIS spectrometer at 460 nm [24].
- The concentration of ammonium was obtained through a potentiometric method, with a selective electrode (Mettler Toledo Type 15 223 3000 Ammonium Electrode) (Columbus, OH, USA) and an Ag/AgCl reference electrode (Mettler Toledo Type 373-90-WTE-ISE-S7) (Columbus, OH, USA) [27].
- SCN− and NH4+ were also measured through ion-exchange chromatography, together other anions such as SO42−, NO3− and Cl−. A Metrohm Ion Chromatograph 850 Professional IC (Metrohm AG, Herisau, Switzerland) was used, equipped with a Metrosep A Supp 5–100 column for anions, a Metrosep C 3–250/4.0 column for cations and a conductivimeter as a detector.
- Heavy metals and other atomic elements were evaluated by inductively coupled plasma (ICP) in an Agilent 7500 ICP-MS (mass spectrometer) device (Agilet, Santa Clara, CA, USA).
3. Results
3.1. Analysis of the Samples
3.2. Effect of Operating Variables with Synthetic Solutions
3.3. Effect of the Transmembrane Pressure with Real Wastewaters
3.4. Effect of Feed Concentration with Real Wastewaters
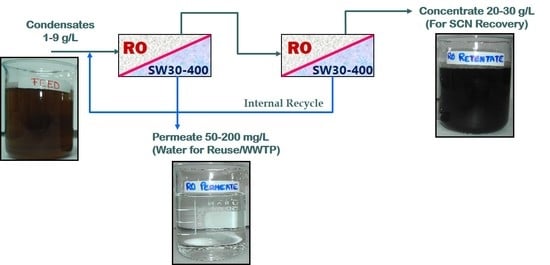
3.5. Design of a RO Plant for Continuous Operation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.M.; Park, D.; Jeon, C.O.; Lee, D.S.; Park, J.M. Effect of HRT on the biological pre-denitrification process for the simultaneous removal of toxic pollutants from cokes wastewater. Biores Technol. 2008, 99, 8824–8832. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-X.; Zhang, Z.-Y.; Fan, Q.-L.; Yuan, X.-Y.; Guo, D.-S. The effect of treatment stages on the coking wastewater hazardous compounds and their toxicity. J. Hazard. Mater. 2012, 239–240, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Staib, C.; Lant, P. Thiocyanate degradation during activated sludge treatment of coke-ovens wastewater. Biochem. Eng. J. 2007, 34, 122–130. [Google Scholar] [CrossRef]
- Balawejdar, E.K. A macromolecular N,N-dichloro-sulfonamide as oxidant for thiocyanates. Eur. Polym. J. 2000, 36, 1137–1143. [Google Scholar] [CrossRef]
- Sharma, V.K.; Burnett, C.R.; O’Connor, D.B.; Cabelli, D. Iron(VI) and iron(V) oxidation of thiocyanate. Environ. Sci. Technol. 2002, 36, 4182–4186. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.N.; Tuan, Y.-J. Chemical Oxidation of Thiocyanate Ion by Ozone. Ozone Sci. Eng. 1993, 15, 343–360. [Google Scholar] [CrossRef]
- Lay-Son, M.; Drakides, C. New approach to optimize operational conditions for the biological treatment of a high-strength thiocyanate and ammonium waste: pH as key factor. Water Res. 2008, 42, 774–780. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Chung, J.S. Biodegradation of thiocyanate in biofilm reactor using fluidized-carriers. Proc. Biochem. 2006, 41, 701–707. [Google Scholar] [CrossRef]
- Hung, C.-H.; Pavlostathis, S.G. Aerobic biodegradation of thiocyanate. Water Res. 1997, 31, 2761–2770. [Google Scholar] [CrossRef]
- Kirk, R.E.; Othmer, D.F. Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons: New York, NY, USA, 1997; Volume 23, p. 322. [Google Scholar]
- Kawasaki, S.; Kohara, N. Process for Recovering Thiocyanate. U.S. Patent Number 5.344.564, 6 September 1994. [Google Scholar]
- Minhalma, M.; de Pinho, M.N. Integration of nanofiltration/steam stripping for the treatment of coke plant ammoniacal wastewaters. J. Membrane Sci. 2004, 242, 87–95. [Google Scholar] [CrossRef]
- Sridhar, S.; Smith, B. New Developments in Nanofiltration Technology: A Case Study on Recovery of Impurity-Free Sodium Thiocyanate for Acrylic Fiber Industry. In Handbook of Membrane Separations. Chemical, Pharmaceutical, Food, and Biotechnological Applications; Pabby, A.K., Rizvi, S.H., Sastre, A.M., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 1101–1129. [Google Scholar]
- Ozyonar, F.; Karagozoglu, B. Treatment of pretreated coke wastewater by electrocoagulation and electrochemical peroxidation processes. Sep. Purif. Technol. 2015, 150, 268–277. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P. A novel forward osmosis-nano filtration integrate system for coke-oven wastewater reclamation. Chem Eng. Res. Des. 2015, 100, 542–553. [Google Scholar] [CrossRef]
- Sridhar, S.; Gorugantu, S.M.; Duraiswamy, S.; Biduru, S.; Machiraju, R. Recovery of Sodium Thiocyanate from Industrial Process Solution using Nanofiltration Technique. U.S. Patent Number 7,314,606, 1 January 2008. [Google Scholar]
- Bódalo-Santoyo, A.; Gómez-Carrasco, J.L.; Gómez-Gómez, E. Application of reverse osmosis to reduce pollutants present in industrial wastewater. Desalination 2003, 155, 101–108. [Google Scholar] [CrossRef]
- Yin, N.; Liu, F.; Zhong, Z.; Xing, W. Integrated Membrane Process for the Treatment of Desulfurization Wastewater. Ind. Eng. Chem. Res. 2010, 49, 3337–3341. [Google Scholar] [CrossRef]
- Yin, N.; Liu, F.; Zhong, Z.; Xing, W. Separation of ammonium salts from coking wastewater with nanofiltration combined with diafiltration. Desalination 2011, 268, 233–237. [Google Scholar] [CrossRef]
- Cho, Y.; Cattrall, R.W.; Kolev, S.D. A novel polymer inclusion membrane based method for continuous clean-up of thiocyanate from gold mine tailings water. J. Hazar. Mater. 2018, 341, 297–303. [Google Scholar] [CrossRef]
- Jin, X.; Li, E.; Lu, S.; Qiu, Z.; Sui, Q. Coking wastewater treatment for industrial reuse purpose: Combining biological processes with ultrafiltration, nanofiltration and reverse osmosis. J. Environ. Sci. 2013, 25, 1565–1574. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Williams, M.E.; Ray, R.J.; McGray, S.B. Theory of Reverse Osmosis. In Membrane Handbook; Ho, W.S., Sirkar, K.K., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1992; pp. 269–280. [Google Scholar]
- Field, R.W. Mass transport and the design of membrane systems. In Industrial Membrane Separation Technology; Scott, K., Hughes, R., Eds.; Blackie Academy and Professionals; Imprint of Chapman and Hall: Glasgow, UK, 1996; pp. 67–113. [Google Scholar]
- American Public Health Association, American Water Works Association, Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater, 17th ed.; Spanish Translation; Ediciones Diaz de Santo: Madrid, Spain, 1992; pp. 2-80–2-85, 4-23–4-43, 4-48–4-50, 5-19. [Google Scholar]
- Knutsson, M.; Jöhnson, J.A. Determination of phenolic compounds in water. In Handbook of Water Analysis; Nollet, L.M.L., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 347–366. [Google Scholar]
- ISO 14403 (2002). Water Quality—Determination of Total Cyanide and Free Cyanide by Continuous Flow Analysis; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- ISO 6778 (1984). Water Quality—Determination of Ammonium—Potentiometric Method; ISO: Geneva, Switzerland, 1984. [Google Scholar]
- Rohman, N.; Wahab, A.; Daas, N.N.; Mahiuddin, S. Viscosity, electrical conductivity, shears relaxation time and Raman spectra of aqueous and methanolic sodium thiocyanate solutions. Fluid Phase Equil. 2001, 178, 277–297. [Google Scholar] [CrossRef]
- Sagiv, A.; Avraham, N.; Dosoretz, C.G.; Semiat, R. Osmotic backwash mechanism of reverse osmosis membranes. J. Membr. Sci. 2008, 322, 225–233. [Google Scholar] [CrossRef]
- Vegas, R.; Moure, A.; Dominguez, H.; Parajo, J.C.; Alvarez, J.R.; Luque, S. Evaluation of ultra- and nanofiltration for refining soluble products from rice husk xylan. Biores. Technol. 2008, 99, 5341–5351. [Google Scholar] [CrossRef]
- Chen, K.L.; Song, L.; Ong, S.L.; Ng, W.J. The development of membrane fouling in full-scale RO processes. J. Membr. Sci. 2004, 232, 63–72. [Google Scholar] [CrossRef]










| Membrane Module | Nominal Active Surface Area (m2) | Maximum Feed Flow Rate (m3/h) | Stabilized Salt Rejection 1 (%) |
|---|---|---|---|
| SW30-2540 | 2.8 | 1.4 | 99.4 |
| SW30-4040 | 7.4 | 3.6 | 99.4 |
| Parameter/Element | Minimum | Maximum | Element | Minimum | Maximum |
|---|---|---|---|---|---|
| pH | 8.6 | 9.1 | Ca (mg/L) | 0.6 | 3.5 |
| Conductivity (mS/cm) | 4.8 | 14.6 | K (mg/L) | 0.6 | 2.7 |
| COD (g/L) | 0.6 | 3.2 | Si (mg/L) | 0.0 | 2.1 |
| SCN− (g/L) | 0.2 | 9.0 | Br (mg/L) | 0.1 | 0.6 |
| NH4+ (g/L) | 1.0 | 12.9 | I (mg/L) | 0.0 | 0.2 |
| Phenols (measured as absorbance at 280 nm) | 1.16 | 3.55 | Sr (mg/L) | 0.0 | 0.1 |
| Al (mg/L) | 0.0 | 0.2 | |||
| SO42− (mg/L) | 130 | 1007 | Cu (mg/L) | 0.0 | 5.0 |
| Cl− (mg/L) | 6 | 50 | Zn (mg/L) | 0.0 | 0.1 |
| NO3− (mg/L) | 12 | 60 | As (mg/L) | 0.0 | 0.1 |
| Fe (mg/L) | 12 | 30 | B (mg/L) | 0.0 | 0.2 |
| Mg (mg/L) | 0.9 | 13.6 | Ba (mg/L) | 0.0 | 0.5 |
| Na (mg/L) | 1.3 | 6.6 | Ge (mg/L) | 0.0 | 0.3 |
| QF (cF) | QP1 (cP1) | QB1 (cB1) | QP2 (cP2) | QB2 (cB2) |
|---|---|---|---|---|
| 2083 (1.0) | 2007 (0.050) | 525 (4.6) | 449 (1.0) | 76 (26.0) |
| 2083 (3.0) | 1750 (0.078) | 1025 (6.3) | 691 (0.5) | 334 (18.0) |
| 2083 (9.0) | 1176 (0.226) | 1525 (12.4) | 617 (0.6) | 907 (20.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, J.R.; Antón, F.E.; Álvarez-García, S.; Luque, S. Treatment of Aqueous Effluents from Steel Manufacturing with High Thiocyanate Concentration by Reverse Osmosis. Membranes 2020, 10, 437. https://doi.org/10.3390/membranes10120437
Álvarez JR, Antón FE, Álvarez-García S, Luque S. Treatment of Aqueous Effluents from Steel Manufacturing with High Thiocyanate Concentration by Reverse Osmosis. Membranes. 2020; 10(12):437. https://doi.org/10.3390/membranes10120437
Chicago/Turabian StyleÁlvarez, José R., F. Enrique Antón, Sonia Álvarez-García, and Susana Luque. 2020. "Treatment of Aqueous Effluents from Steel Manufacturing with High Thiocyanate Concentration by Reverse Osmosis" Membranes 10, no. 12: 437. https://doi.org/10.3390/membranes10120437
APA StyleÁlvarez, J. R., Antón, F. E., Álvarez-García, S., & Luque, S. (2020). Treatment of Aqueous Effluents from Steel Manufacturing with High Thiocyanate Concentration by Reverse Osmosis. Membranes, 10(12), 437. https://doi.org/10.3390/membranes10120437