PVDF-Modified Nafion Membrane for Improved Performance of MFC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition of the Experimental System
2.2. Experimental Materials and Pretreatment
2.3. Preparation of Membranes
2.4. Analysis Method
3. Results and Discussion
3.1. Comparison of Power Generation Performance
3.2. SEM Analysis
3.3. Hydroscopicity Analysis of Membrane
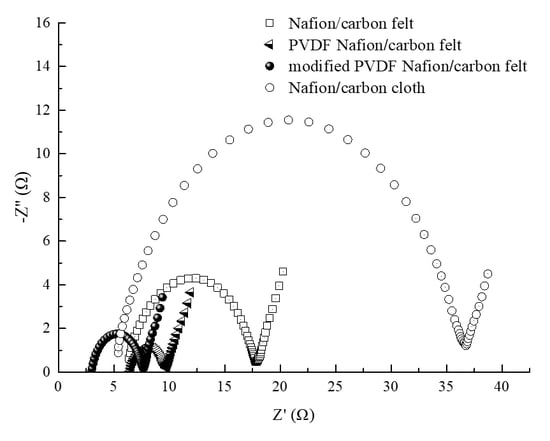
3.4. Analysis of EIS
3.5. Water Quality Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Pagliano, G.; Ventorino, V.; Pánico, A.; Pepe, O. Integrated systems for biopolymers and bioenergy production from organic waste and by-products: A review of microbial processes. Biotechnol. Biofuels 2017, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Kumar, V.; Malyan, S.K.; Sharma, J.; Mathimani, T.; Maskarenj, M.S.; Ghosh, P.C.; Pugazhendhi, A. Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel 2019, 254, 115526. [Google Scholar] [CrossRef]
- Sreelekshmy, B.R.; Basheer, R.; Sivaraman, S.; Vasudevan, V.; Elias, L.; Shibli, S.; Savithri, S.; Lal, V. Sustainable electric power generation from live anaerobic digestion of sugar industry effluents using microbial fuel cells. J. Mater. Chem. A 2020, 8, 6041–6056. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, S.; Jia, L.; Ji, A.; Chatterjee, S.G. Co-generation system of bioethanol and electricity with microbial fuel cell technology. Energy Fuels 2020, 34, 6414–6422. [Google Scholar] [CrossRef]
- Liu, F.; Sun, L.; Wan, J.; Tang, A.; Deng, M.; Wu, R. Organic matter and ammonia removal by a novel integrated process of constructed wetland and microbial fuel cells. RSC Adv. 2019, 9, 5384–5393. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.S.; Kumar, V.; Kumar, R.; Malyan, S.K.; Pugazhendhi, A. Microbial fuel cells as a sustainable platform technology for bioenergy, biosensing, environmental monitoring, and other low power device applications. Fuel 2019, 255, 115682. [Google Scholar] [CrossRef]
- Fan, L.P.; Zheng, Y.J.; Miao, X.H. Effects of catholyte and dissolved oxygen on microbial fuel cell performance. J. Chem. Eng. Chin. Univ. 2016, 30, 491–496. [Google Scholar]
- Penteado, E.D.; Fernandez-Marchante, C.M.; Zaiat, M.; Gonzalez, E.R.; Rodrigo, M.A. Optimization of the performance of a microbial fuel cell using the ratio electrode-surface area/anode compartment. Braz. J. Chem. Eng. 2018, 35, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.P.; Miao, X.H. Study on the performance of microbial fuel cell for restaurant wastewater treatment and simultaneous electricity generation. J. Fuel Chem. Technol. 2014, 42, 1506–1512. [Google Scholar]
- Yang, Y.; Yan, L.; Song, J.; Xu, M. Optimizing the electrode surface area of sediment microbial fuel cells. RSC Adv. 2018, 8, 25319–25324. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.P.; Bao, W.F. Effects of K2Cr2O7 and KClO3 modified anode on MFC performance. J. Chem. Eng. Chin. Univ. 2019, 33, 758–764. [Google Scholar]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Environmentally sustainable biogenic fabrication of AuNP decorated-graphitic g-C3N4 nanostructures towards improved photoelectrochemica performances. RSC Adv. 2018, 8, 13898–13909. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.E.; Han, T.H.; Khan, M.M.; Karim, R.; Cho, M.H. Environmentally sustainable fabrication of Ag@g-C3N4 nanostructures and their multifunctional efficacy as antibacterial agents and photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2912–2922. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, S.; Zhang, L.; He, M. A facile synthesis of molybdenum carbide nanoparticles-modified carbonized cotton textile as an anode material for high-performance microbial fuel cells. RSC Adv. 2018, 8, 40490–40497. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Thiruvenkadam, S.; Prasad, R.; Khan, M.R. Enhanced current generation using mutualistic interaction of yeast-bacterial coculture in dual chamber microbial fuel cell. Ind. Eng. Chem. Res. 2018, 57, 813–821. [Google Scholar] [CrossRef]
- Liu, J.; Tian, C.; Xiong, J.; Wang, L. Polypyrrole blending modification for PVDF conductive membrane preparing and fouling mitigation. J. Colloid Interface Sci. 2017, 494, 124–129. [Google Scholar] [CrossRef]
- Kong, X.; Sun, Y.; Li, L.; Li, Y.; Yuan, Z. Electricity generation comparison of two-chamber microbial fuel cells with different membrane. Acta Energy Sol. Sin. 2012, 33, 882–887. [Google Scholar]
- Hernandez-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; Romero-Castañón, T.; Ríos-Leal, E.; Galindez-Mayer, J.; Esparza-García, F. Batch operation of a microbial fuel cell equipped with alternative proton exchange membrane. Int. J. Hydrog. Energy 2015, 40, 17323–17331. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; Pérezde los Ríos, A.; Mateo-Ramírez, F.; Godínez, C.; Lozano-Blanco, L.J.; Moreno, J.I.; Tomás-Alonso, F. New application of supported ionic liquids membranes as proton exchange membranes in microbial fuel cell for waste water treatment. Chem. Eng. J. 2015, 279, 115–119. [Google Scholar] [CrossRef]
- Fan, L.P.; Zhang, L.L. Effect of heteropolyacid and heteropolyacid salt on the performance of nanometer proton membrane microbial fuel cell. Int. J. Electrochem. Sci. 2017, 12, 699–709. [Google Scholar] [CrossRef]
- Ma, Z.; Lu, X.; Wu, C.; Gao, Q.; Zhao, L.; Zhang, H.; Liu, Z. Functional surface modification of PVDF membrane for chemical pulse cleaning. J. Membr. Sci. 2017, 524, 389–399. [Google Scholar] [CrossRef]
- Sun, C.; Feng, X. Enhancing the performance of PV DF membranes by hydrophilic surface modification via amine treatment. Sep. Purif. Technol. 2017, 185, 94–102. [Google Scholar] [CrossRef]
- Sun, J.; Li, S.; Ran, Z.; Xiang, Y. Preparation of Fe3O4@TiO2 blended PVDF membrane by magnetic coagulation bath and itspermeability and pollution resistance. J. Mater. Res. Technol. 2020, 9, 4951–4967. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Gao, T. Comparative study on the effects of three membrane modification methods on the performance of microbial fuel cell. Energies 2020, 13, 1383. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Ning, J.; Jiang, F.; Shi, J.; Huang, F. Ceramic nanoparticle-decorated melt-electrospun PVDF nanofiber membrane with enhanced performance as a Lithium-Ion Battery Separator. ACS Omega 2019, 4, 16309–16317. [Google Scholar] [CrossRef] [Green Version]
- Ekambaram, K.; Doraisamy, M. Surface modification of PVDF nanofiltration membrane using Carboxymethylchitosan-Zinc oxide bionanocomposite for the removal of inorganic salts and humic acid. Colloids Surf. A 2017, 525, 49–63. [Google Scholar] [CrossRef]
- Daud, S.M.; Daud, W.R.W.; Kim, B.H. Comparison of performance and ionic concentration gradient of twochamber microbial fuel cell using ceramic membrane (CM) and cation exchange membrane (CEM) as separators. Electrochim. Acta 2018, 259, 365–376. [Google Scholar] [CrossRef]
- Dunaj, S.J.; Vallino, J.; Hines, M.E.; Gay, M.; Kobyljanec, C.; Rooney-Varga, J.N. Relationships between Soil Organic Matter, Nutrients, Bacterial Community Structure, and the Performance of Microbial Fuel Cells. Environ. Sci. Technol. 2012, 46, 1914–1922. [Google Scholar] [CrossRef]
- Islam, A.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Khan, M.R. Electrogenic and antimethanogenic properties of bacillus cereus for enhanced power generation in anaerobic sludge-driven microbial fuel cells. Energy Fuels 2017, 31, 6132–6139. [Google Scholar] [CrossRef]
- Heravi, M.M.; Ghavidel, M.; Mohammadkhani, L. Beyond a solvent: Triple roles of dimethylformamide in organic chemistry. RSC Adv. 2018, 8, 27832–27862. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Wycisk, R.; Pintauro, P.N. Nafion/PVDF nanofiber composite membranes for regenerative hydrogen/bromine fuel cells. J. Membr. Sci. 2015, 490, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.N. Study on methods for analyzing the stability of satellite navigation signals. Astron. Res. Technol. 2017, 14, 330–336. [Google Scholar]
- Liu, W.; Du, J.P.; Zhou, P.F.; Ma, T. Research on fault diagnosis of power battery for electric vehicle based on fuzzy algorithm. Bus Coach Technol. Res. 2018, 40, 9–13. [Google Scholar]
- Vinothkannan, M.; Kim, A.; Kumar, G.G.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef] [Green Version]
- Mathuriya, A.S.; Pant, D. Assessment of expanded polystyrene as a separator in microbial fuel cell. Environ. Technol. 2019, 40, 2052–2061. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Ma, Y.; Li, T.; Dong, Z.S.; Wang, Y.X. Modification of carbon felt anodes using doubleoxidant HNO3/H2O2 for application in microbial fuel cells. RSC Adv. 2018, 8, 2059–2064. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Zhang, D.; You, H.; Li, W.; Jiang, K. Benthic microbial fuel cell equipped with a photocatalytic Cu2O-coated cathode. J. Nanopart. Res. 2019, 21, 3. [Google Scholar] [CrossRef]
- Sarmin, S.; Ethiraj, B.; Islam, A.; Ideris, A.; Yee, C.S.; Khan, M.R.; Khan, M.R. Bio-electrochemical power generation in petrochemical wastewater fed microbial fuel cell. Sci. Total Environ. 2019, 695, 133820. [Google Scholar] [CrossRef]
- Leuaa, P.; Priyadarshani, D.; Tripathi, A.K.; Neergat, M. Internal and external transport of redox species across the porous thin-film electrode/electrolyte interface. J. Phys. Chem. C 2019, 123, 21440–21447. [Google Scholar] [CrossRef]
- Ma, C.; Wen, H.; Xing, D.; Pei, X.; Zhu, J.; Ren, N.; Liu, B. Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus sp. Z-4. Biotechnol. Biofuels 2017, 10, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apollo, S.; Onyongo, M.S.; Ochieng, A. UV/H2O2/TiO2/Zeolite hybrid system for treatment of molasses wastewater. Iran. J. Chem. Chem. Eng. 2014, 33, 107–117. [Google Scholar]
- Sirianuntapiboon, S.; Phothilangka, P.; Ohmomo, S. Decolorization of molasses wastewater by a strain No. BP103 of acetogenic bacteria. Bioresour. Technol. 2004, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]







| Nafion/Carbon Cloth | Nafion/Carbon Felt | PVDF–Nafion/Carbon Felt | Modified PVDF–Nafion/Carbon Felt | |
|---|---|---|---|---|
| Steady-state value (A/m2) | 0.062 | 0.181 | 0.219 | 0.233 |
| Standard deviation (A/m2) | 0.023 | 0.037 | 0.062 | 0.013 |
| Nafion/Carbon Felt | PVDF–Nafion/Carbon Felt | Modified PVDF–Nafion/Carbon Felt | |
|---|---|---|---|
| Wet weight W1 (g) | 3.65 | 3.92 | 3.87 |
| Dry weight W0 (g) | 3.43 | 3.50 | 3.46 |
| Water absorption rate ΔW (%) | 6.41 | 12.00 | 11.80 |
| Nafion/Carbon Felt | PVDF–Nafion/Carbon Felt | Modified PVDF–Nafion/Carbon Felt | Nafion/Carbon Cloth | |
|---|---|---|---|---|
| Rs/Ω | 6.191 | 6.518 | 3.162 | 4.977 |
| Rct/Ω | 11.36 | 2.897 | 4.138 | 31.38 |
| Zw/(Ω) | 0.7962 | 0.6684 | 0.8775 | 0.7758 |
| Nafion | PVDF–Nafion | Modified PVDF–Nafion | |
|---|---|---|---|
| Influent (mg/L) | 3663 | 3663 | 3663 |
| Effluent (mg/L) | 2266 | 1354 | 1220 |
| COD removal rate (%) | 38.1 | 63.0 | 66.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Shi, J.; Xi, Y. PVDF-Modified Nafion Membrane for Improved Performance of MFC. Membranes 2020, 10, 185. https://doi.org/10.3390/membranes10080185
Fan L, Shi J, Xi Y. PVDF-Modified Nafion Membrane for Improved Performance of MFC. Membranes. 2020; 10(8):185. https://doi.org/10.3390/membranes10080185
Chicago/Turabian StyleFan, Liping, Junyi Shi, and Yaobin Xi. 2020. "PVDF-Modified Nafion Membrane for Improved Performance of MFC" Membranes 10, no. 8: 185. https://doi.org/10.3390/membranes10080185
APA StyleFan, L., Shi, J., & Xi, Y. (2020). PVDF-Modified Nafion Membrane for Improved Performance of MFC. Membranes, 10(8), 185. https://doi.org/10.3390/membranes10080185