Facilitated Transport of Copper(II) across Polymer Inclusion Membrane with Triazole Derivatives as Carrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of 1-alkyl-1,2,4-triazole
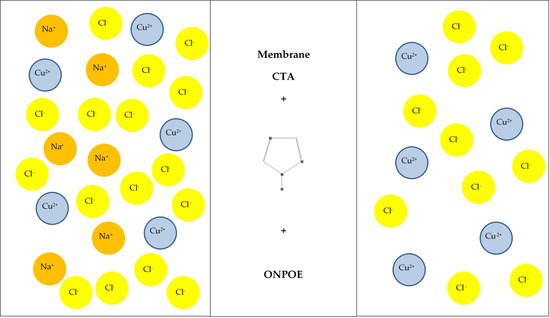
2.3. Membrane Preparation
2.4. Transport Experiment
3. Results and Discussion
3.1. Influence of Volumetric Flow Rate of Phases on Transport of Cu(II) Ions
3.2. Chloride Anion Transport
3.3. Influence of Concentration of Chloride Anions on Transport of Cu(II) Through PIM
3.4. Influence of The Length of The Alkyl Ligand
3.5. Temperature Effect
3.6. Separation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Habashi, F. Handbook of Extractive Metallurgy; WILEY-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Biswas, A.K.; Davenport, W.G. Extractive Metallurgy of Copper, 3rd ed.; Pergamon: Oxford, UK, 1994. [Google Scholar]
- Kavitha, N.; Palanivelu, K. Recovery of copper(II) through polymer inclusion membrane with di (2-ethylhexyl) phosphoric acid as carrier from e-waste. J. Membr. Sci. 2012, 415–416, 663–669. [Google Scholar] [CrossRef]
- Carranza, F.; Iglesias, N.; Mazuelos, A.; Palencia, I.; Romero, R. Treatment of copper concentrates containing chalcopyrite and non-ferrous sulphides by the BRISA process. Hydrometallurgy 2004, 71, 413–420. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Panias, D. Differential precipitation of copper and nickel from acidic polymetallic aqueous solutions. Hydrometallurgy 2008, 90, 137–146. [Google Scholar] [CrossRef]
- Morin, D.; Pinches, T.; Huisman, J.; Frias, C.; Norberg, A.; Forssberg, E. Progress after three years of BioMinE-Research and Technological Development project for a global assessment of biohydrometallurgical processes applied to European non-ferrous metal resources. Hydrometallurgy 2008, 94, 58–68. [Google Scholar] [CrossRef]
- Jorjani, E.; Ghahreman, A. Challenges with elemental sulfur removal during the leaching of copper and zinc sulfides, and from the residues; a review. Hydrometallurgy 2017, 171, 333–343. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.W. Microwave-assisted leaching—A review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Arai, M.; Miki, H.; Tsunekawa, M.; Hirajima, T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2002, 63, 257–267. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Gallardo, E.; Padilla, R. Copper extraction from white metal by pressure leaching in H2SO4-FeSO4-O2. Hydrometallurgy 2009, 100, 50–55. [Google Scholar] [CrossRef]
- Vinals, J.; Fuentes, G.; Hernández, M.C.; Herreros, O. Transformation of sphalerite particles into copper sulfide particles by hydrothermal treatment with Cu(II) ions. Hydrometallurgy 2004, 75, 177–187. [Google Scholar] [CrossRef]
- Jin, B.; Yang, X.; Shen, Q. Kinetics of copper dissolution during pressure oxidative leaching of lead-containing copper matte. Hydrometallurgy 2009, 99, 119–123. [Google Scholar] [CrossRef]
- Gunn, G. Critical Metals Handbook; John Wiley and Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Anjum, F.; Shahid, M.; Akcil, A. Biohydrometallurgy techniques of low grade ores: A review on black shale. Hydrometallurgy 2012, 117–118, 1–12. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Agarwal, S.; Machado, R.M.; Gameiro, M.L.; Santos, S.M.C.; Reis, M.T.; Ismael, M.R.; Correia, M.J.; Carvalho, J.M.R. Extraction of copper from acidic leach solution with Acorga M5640 using a pulsed sieve plate column. Hydrometallurgy 2010, 104, 66–75. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Munoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Petranikova, M.; Herdzik-Koniecko, I.; Steenari, B.M.; Ekberg, C. Hydrometallurgical processes for recovery of valuable and critical metals from spent car NiMH batteries optimized in a pilot plant scale. Hydrometallurgy 2017, 171, 128–141. [Google Scholar] [CrossRef]
- Ilyas, S.; Ruan, C.; Bhatti, H.N.; Ghauri, M.A.; Anwar, M.A. Column bioleaching of metals from electronic scrap. Hydrometallurgy 2010, 101, 135–140. [Google Scholar] [CrossRef]
- Liang, C.L.; Xia, J.L.; Zhao, X.J.; Yang, Y.; Gong, S.Q.; Nie, Z.Y.; Ma, C.Y.; Zheng, L.; Zhao, Y.D.; Qiu, G.z. Effect of activated carbon on chalcopyrite bioleaching with extreme thermophile Acidianus manzaensis. Hydrometallurgy 2010, 105, 179–185. [Google Scholar] [CrossRef]
- Banza, A.N.; Gock, E.; Kongolo, K. Base metals recovery from copper smelter slag by oxidising leaching and solvent extraction. Hydrometallurgy 2002, 67, 63–69. [Google Scholar] [CrossRef]
- Palencia, I.; Romero, R.; Mazuelos, A.; Carranza, F. Treatment of secondary copper sulphides (chalcocite and covellite) by the BRISA process. Hydrometallurgy 2002, 66, 85–93. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Past, present and future of biohydrometallurgy. Hydrometallurgy 2001, 59, 127–134. [Google Scholar] [CrossRef]
- Dreisinger, D. Copper leaching from primary sulfides: Options for biological and chemical extraction of copper. Hydrometallurgy 2006, 83, 10–20. [Google Scholar] [CrossRef]
- Carneiro, M.F.C.; Leao, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Dreisinger, D.; Abed, N. A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: Kinetic analysis. Hydrometallurgy 2002, 66, 37–57. [Google Scholar] [CrossRef]
- Godočíková, E.; Baláž, P.; Boldížárová, E. Structural and temperature sensitivity of the chloride leaching of copper, lead and zinc from a mechanically activated complex sulphide. Hydrometallurgy 2002, 65, 83–93. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Munoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential. Hydrometallurgy 2008, 93, 88–96. [Google Scholar] [CrossRef]
- Fuentes, G.; Vinals, J.; Herreros, O. Hydrothermal purification and enrichment of Chilean copper concentrates. Part 2: The behavior of the bulk concentrates. Hydrometallurgy 2009, 95, 113–120. [Google Scholar] [CrossRef]
- Hou, X.; Xiao, L.; Gao, C.; Zhang, Q.; Zeng, L. Kinetics of leaching selenium from Ni-Mo ore smelter dust using sodium chlorate in a mixture of hydrochloric and sulfuric acids. Hydrometallurgy 2010, 104, 76–80. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Sánchez-Castellanos, M.; Rodríguez de San Miguel, E.; de Gyves, J. Cd(II) and Pb(II) extraction and transport modeling in SLM and PIM systems using Kelex 100 as carrier. J. Membr. Sci. 2001, 190, 107–118. [Google Scholar] [CrossRef]
- Tasaki, T.; Oshima, T.; Baba, Y. Selective extraction and transport of copper(II) with new alkylated pyridinecarboxylic acid derivatives. Talanta 2007, 73, 387–393. [Google Scholar] [CrossRef]
- Ansari, S.A.; Mohapatra, P.K.; Manchanda, V.K. Cation transport across plasticized polymeric membranes containing N,N,N’,N’-tetraoctyl-3-oxapentanediamide(TODGA) as the carrier. Desalination 2010, 262, 196–201. [Google Scholar] [CrossRef]
- De Gyves, J.; Hernández-Andaluz, A.M.; Rodríguez de San Miguel, E. LIX®-loaded polymer inclusion membrane for copper(II) transport: 2. Optimization of the efficiency factors (permeability, selectivity, and stability) for LIX® 84-I. J. Membr. Sci. 2006, 268, 142–149. [Google Scholar] [CrossRef]
- Rodríguez de San Miguel, E.; Hernández-Andaluz, A.M.; Banuelos, J.G.; Saniger, J.M.; Aguilar, J.C.; de Gyves, J. LIX®-Loaded polymer inclusion membrane for copper(II) transport: 1. Composition-performance relationships through membrane characterization and solubility diagrams. Mater. Sci. Eng. A 2006, 434, 30–38. [Google Scholar] [CrossRef]
- Kordosky, G.A. Copper recovery using leach/solvent extraction/electrowinning technology: Forty years of innovation, 2.2 million tonnes of copper annually. J. S. Afr. Inst. Min. Metall. 2002, 102, 445–450. [Google Scholar]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported liquid membrane and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Kumar, R.; Pandey, A.K.; Sharma, M.K.; Panicker, L.V.; Sodaye, S.; Suresh, G.; Ramagiri, S.V.; Bellare, J.R.; Goswami, A. Diffusional transport of ions in plasticized anion-exchange membranes. J. Phys. Chem. B 2011, 115, 5856–5867. [Google Scholar] [CrossRef] [PubMed]
- Pośpiech, B.; Walkowiak, W. Separation of copper(II), cobalt(II) and nickel(II) from chloride solutions by polymer inclusion membranes. Sep. Purif. Technol. 2007, 57, 461–465. [Google Scholar] [CrossRef]
- Andanson, J.M.; Papaiconomou, N.; Cable, P.A.; Traikia, M.; Billard, I.; Husson, P. The role of association of ions in ionic liquid/molecular solvent mixtures on metal extraction. Phys. Chem. Chem. Phys. 2017, 19, 28834–28840. [Google Scholar] [CrossRef] [Green Version]
- Vander Hoogerstraete, T.; Blockx, J.; De Coster, H.; Binnemans, K. Selective Single-Step Separation of a Mixture of Three Metal Ions by a Triphasic Ionic-Liquid-Water-Ionic-Liquid Solvent Extraction System. Chem. Eur. J. 2015, 21, 11757–11766. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Kubota, F.; Kamiya, N.; Goto, M. A Comparative Study of Ionic Liquids and a Conventional Organic Solvent on the Extraction of Rare-earth Ions with TOPO. Solv. Extr. Res. Dev. Jpn. 2013, 20, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Kumano, M.; Yabutani, T.; Motonaka, J.; Mishima, Y.; Mishima, Y. Recovery and extraction of heavy metal ions using ionic liquid as green solvent. Int. J. Mod. Phys. B 2006, 20, 4051–4056. [Google Scholar] [CrossRef]
- Pośpiech, B. Studies on extraction and permeation of cadmium(II) using Cyphos IL 104 as selective extractant and ion carrier. Hydrometallurgy 2015, 154, 88–94. [Google Scholar] [CrossRef]
- Baczyńska, M.; Regel-Rosocka, M.; Coll, M.T.; Fortuny, A.; Sastre, A.M.; Wiśniewski, M. Transport of Zn(II), Fe(II), Fe(III) across polymer inclusion membranes (PIM) and flat sheet supported liquid membranes (SLM) containing phosphonium ionic liquids as metal ion carriers. Sep. Sci. Technol. 2016, 51, 2639–2648. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Staszak, K.; Wieszczycka, K.; Masalska, A. Removal of cobalt(II) and zinc(II) from sulphate solutions by means of extraction with sodium bis(2,4,4-trimethylpentyl)phosphinate (Na-Cyanex 272). Clean Technol. Environ. Policy 2016, 18, 1961–1970. [Google Scholar] [CrossRef] [Green Version]
- Rout, A.; Binnemans, K. Influence of the ionic liquid cation on the solvent extraction of trivalent rare-earth ions by mixtures of Cyanex 923 and ionic liquids. Dalton Trans. 2015, 44, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Raiguel, S.; Depuydt, D.; Vander Hoogerstraete, T.; Thomas, J.; Dehaen, W.; Binnemans, K. Selective alkaline stripping of metal ions after solvent extraction by base-sTable 1,2,3-triazolium ionic liquids. Dalton Trans. 2017, 46, 5269–5278. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, J.G.H.; Sumter, N.; Mattheüs, C.; Ravindran, S.; Van Brecht, B.J. Nitrogen reagents in metal ion separation. Part VII. The development of a novel copper(II) extractant. Solv. Extr. Ion Exch. 1997, 15, 1007–1021. [Google Scholar] [CrossRef]
- Du Preez, J.G.H. Recent advances in amines as separating agents for metal ions. Solv. Extr. Ion Exch. 2000, 18, 679–701. [Google Scholar] [CrossRef]
- Du Preez, J.G.H.; Mattheüs, C.; Sumter, N.; Ravindran, S.; Potgieter, C.; Van Brecht, B.J. Nitrogen reagents in metal ion separation. Part VIII. Substituted imidazoles as extractants for Cu2+. Solv. Extr. Ion Exch. 1998, 16, 565–586. [Google Scholar] [CrossRef]
- Du Preez, J.G.H.; Mattheüs, C.; Sumter, N.; Edge, W.; Potgieter, C.; Van Brecht, B.J. Nitrogen reagents in metal ion separation. Part IX. Extraction of cobalt and nickel using imidazole derivatives. Solv. Extr. Ion Exch. 1998, 16, 1033–1046. [Google Scholar] [CrossRef]
- Gajda, B.; Skrzypczak, A.; Bogacki, M.B. Separation of Cobalt(II), Nickel(II), Zinc(II) and Cadmium(II) Ions from Chloride Solution. Physicochem. Probl. Miner. Process. 2011, 46, 289–294. [Google Scholar]
- Gajda, B.; Bogacki, M.B. The application of polymer inclusive membranes for removal of heavy metal ions from waste solutions. J. Achiev. Mater. Manuf. Eng. 2012, 55, 673–678. [Google Scholar]
- Ulewicz, M.; Radzymińska-Lenarcik, E. Transport of metal ions across polymer inclusion membrane with 1-alkylimidazole. Physicochem. Probl. Miner. Process. 2011, 46, 119–130. [Google Scholar]
- Radzymińska-Lenarcik, E. Effect of alkyl chain length on the extraction of copper(II) complexes with 1-alkyl-2-methylimidazoles. Sep. Sci. Technol. 2007, 42, 2661–2675. [Google Scholar] [CrossRef]
- Lenarcik, B.; Głowacki, J. Search for Optimum Conditions of Extraction of Transition Metal Complexes with Alkylimidazoles. II. Extraction of the Co(ll), Ni(ll), and Zn(II) Complexes of l-Ethylimidazole and l-Butylimidazole. Sep. Sci. Technol. 1979, 14, 721–734. [Google Scholar] [CrossRef]
- Du Preez, J.G.H.; Gerber, T.I.A.; Edge, W.; Mtotywa, V.L.V.; Van Brecht, B.J.A.M. Nitrogen reagents in metal ion separation. Part XI. The systhesis and extraction behavior of a new imidazole derivative. Solvent Extr. Ion Exch. 2001, 19, 143–154. [Google Scholar] [CrossRef]
- Gabryszewski, M. The complexing behaviour of some alkyl and amino derivatives of 1,2,4-triazole in aqueous solution. Pol. J. Chem. 1992, 66, 1067–1075. [Google Scholar]
- Radzymińska-Lenarcik, E.; Lenarcik, B. Determintion of stability constants of Cu(II) complexes with 1-alkyl-1,2,4-triazoles by liquid-liquid partition method. In Proceedings of the XIX-th International Symposium on Physicochemical Methods of Separation, “Ars Separatoria 2004”, Złoty Potok n, Częstochowa, Poland, 2–5 June 2004; 236 - 238. [Google Scholar]
- Gajewski, P.; Bogacki, M.B. Influence of Alkyl Chain Length in 1-Alkylimidazol on the Citric Acid Transport Rate across Polymer Inclusion Membrane. Sep. Sci. Technol. 2012, 47, 1374–1382. [Google Scholar] [CrossRef]
- Gajewski, P.; Przewoźna, M.; Bogacki, M.B. Influence of Carrier Concentration (1-Alkylimidazols and TOA) on Citric Acid Transport across Polymer Inclusion Membranes (PIM). Sep. Sci. Technol. 2014, 49, 1736–1744. [Google Scholar] [CrossRef]
- Przewoźna, M.; Gajewski, P.; Michalak, N.; Bogacki, M.B.; Skrzypczak, A. Determination of the Percolation Threshold for the Oxalic, Tartaric, and Lactic Acids Transport through Polymer Inclusion Membranes with 1-Alkylimidazoles as a Carrier. Sep. Sci. Technol. 2014, 49, 1745–1755. [Google Scholar] [CrossRef]
- Song, M.K.; Zhu, X.; Liu, M. A triazole-based polymer electrolyte membrane for fuel cells operated in a wide temperature range (25–150°C) with little humidification. J. Power Sources 2013, 241, 219–224. [Google Scholar] [CrossRef]
- Lokesh, S.V.; Satpati, A.K.; Sherigara, B.S. Electrochemical behaviour of 1,2,4-triazole and benzotriazole at glass carbon electrode in acidic media. Open Electrochem. J. 2017, 2, 15–21. [Google Scholar] [CrossRef]
- Fox, P.G.; Bradley, P.A. 1:2:4-triazole as a corrosion inhibitor for copper. Corros. Sci. 1980, 20, 643–649. [Google Scholar] [CrossRef]
- Ramesh, S.; Rajeswari, S.; Maruthamuthu, S. Corrosion inhibition of copper by new triazole phosphonate derivatives. Appl. Surf. Sci. 2004, 229, 214–225. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kurdziel, K.; Gabryszewski, M. Stability and structure of transition metal complexes with azoles in aqueous solution-XXII. Complexing behaviour of 1,2,4-triazole, 3-amino-1,2,4-triazole and 4-amino-1,2,4-triazole. J. Inorg. Nucl. Chem. 1980, 42, 587–592. [Google Scholar] [CrossRef]
- Lenarcik, B.; Ojczenasz, P. The influence of the size and position of the alkyl groups in alkylimidazole molecules on their acid-base properties. J. Heterocycl. Chem. 2002, 39, 287–290. [Google Scholar] [CrossRef]
- Okewole, A.I.; Walmsley, R.S.; Valtancoli, B.; Bianchi, A.; Tshentu, Z.R. Separation of Copper(II) from Base Metals in an Acidic Synthetic Sulfate Leach Solution Using a Novel 1-Octylimidazole-2-Aldoxime Extractant. Solv. Extr. Ion Exch. 2013, 31, 61–78. [Google Scholar] [CrossRef]
- Okewole, A.I.; Magwa, N.P.; Tshentu, Z.R. The separation of nickel(II) from base metal ions using 1-octyl-2-(2-pyridyl)imidazole as extractant in a highly acidic sulfate medium. Hydrometallurgy.
- Radzymińska-Lenarcik, E.; Ulewicz, M. Selective Transport of Cu(II) across a Polymer Inclusion Membrane with 1-Alkylimidazole from Nitrate Solutions. Sep. Sci. Technol. 2012, 47, 1113–1118. [Google Scholar] [CrossRef]
- Lenarcik, B.; Rauckyte, T.; Kopkowski, A. Application of extraction method to the investigation of the stability constants of Ni(II) complexes with 1-alkyl-1,2,4-triazoles by using several organic solvents. In Proceedings of the XVII International Symposium on Physicochemical Methods of Separation, “Ars Separatoria 2002”, Borówno, Poland, 17–20 June 2002. [Google Scholar]
- Daily, L.A.; Miller, K.M. Correlating Structure with Thermal Properties for a Series of 1-Alkyl-4-methyl-1,2,4-triazolium Ionic Liquids. J. Org. Chem. 2013, 78, 4196–4201. [Google Scholar] [CrossRef]
- Zaki, M.T.M.; Alqasmi, R. Spectrophotometric determination of copper(II) as citrate or EDTA complex. Fresenius Z. Anal. Chem. 1981, 306, 400. [Google Scholar] [CrossRef]
- Ellison, S.L.R.; Williams, A. (Eds.) Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, 3rd ed.; Eurachem: London, UK, 2012. [Google Scholar]
- St John, A.M.; Cattrall, R.W.; Kolev, S.D. Determination of the initial flux of polymer inclusion membranes. Sep. Purif. Technol. 2013, 116, 41–45. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of Metal Species by Supported Liquid Membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Sarmiento, A.P.C.; Soares, V.H.T.; Milanez, F.H.; Mantelli, M.B.H. Heat transfer correlation for circular and non-circular ducts in the transition regime. Int. J. Heat Mass Transf. 2020, 149, 119165–119184. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E.J. Membrane Separations. In Separation Process Principles; Seader, J.D., Henley, E.J., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 1998; pp. 713–777. [Google Scholar]
- Rydberg, J.; Sekine, T. Solvent extraction equilibria. In Principles and Practice of Solvent Extraction; Rydberg, J., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1992; pp. 101–156. [Google Scholar]
- Catalan, J.; Claramunt, R.M.; Elguero, J.; Laynez, J.; Menendez, M.; Anvia, F.; Quian, J.H.; Taagepera, M.; Taft, R.W. Basicity and acidity of azoles: The annelation effect in azoles. J. Am. Chem. Soc. 1988, 110, 4105–4111. [Google Scholar] [CrossRef]
- Haasnoot, J.G. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands. Coord. Chem. Rev. 2000, 200–202, 131–185. [Google Scholar] [CrossRef]
- Zaydoun, S.; Idrissi, M.S.; Zrineh, A.; Agricole, B.; Garrigou-Lagranage, C. Transition metal complexes of 1,2,4-triazole: Electronic and magnetic studies. Polyhedron 1995, 14, 1477–1486. [Google Scholar] [CrossRef]
- Högfeldt, E. Stability Constants of Metal—Ion Complexes. Part A: Inorganic Ligands; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Man-Seung, L.; Young-Joo, O. Chemical Equilibria in a Mixed Solution of Nickel and Cobalt Chloride. Mater. Trans. 2005, 46, 59–63. [Google Scholar]
- Ramette, R.W.; Fan, G. Copper(II) chloride complex equilibrium constants. Inorg. Chem. 1983, 22, 3323–3326. [Google Scholar] [CrossRef]
- Ramette, R.W. Copper(II) complexes with chloride ion. Inorg. Chem. 1986, 25, 2481–2482. [Google Scholar] [CrossRef]
- Bjerrum, J.; Skibsted, L.H. Weak chloro complex formation by copper(II) in aqueous chloride solutions. Inorg. Chem. 1986, 25, 2479–2481. [Google Scholar] [CrossRef]
- Bjerrum, J.; Skibsted, L.H. A contribution to our knowledge of weak chloro complex formation by copper(II) in aqueous chloride solutions. Acta Chem. Scand. A 1977, 31, 673–677. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Schwing-Weill, M.J. Stability and electronic spectra of the copper(II) chloro complexes in aqueous solutions. Inorg. Chem. 1976, 15, 2202–2205. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Noble, R.D. Generalized microscopic mechanism of facilitated transport in fixed site carrier membranes. J. Membr. Sci. 1992, 75, 121–129. [Google Scholar] [CrossRef]
- Cussler, E.L.; Aris, R.; Bhown, A. On the limits of facilitated diffusion. J. Membr. Sci. 1989, 43, 149–164. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; de San Miguel, E.R.; Muhammed, M.; de Gyves, J. Transport characterisation of a PIM system used for the extraction of Pb(II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Laidler, K.J.; King, M.C. Development of transition-state theory. J. Phys. Chem. 1983, 87, 2657–2664. [Google Scholar] [CrossRef]
- Fontás, C.; Tayeb, R.; Dhahbi, M.; Gaudichet, E.; Thominette, F.; Roy, P.; Steenkeste, K.; Fontaine-Aupart, M.P.; Tingry, S.; Tronel-Peyroz, E.; et al. Polymer inclusion membranes: The concept of fixed sites membrane revised. J. Membr. Sci. 2007, 290, 62–72. [Google Scholar] [CrossRef]
- Kozłowski, C.A. Kinetics of Chromium(VI) Transport from Mineral Acids across Cellulose Triacetate (CTA) Plasticized Membranes Immobilized by Tri-n-octylamine. Ind. Eng. Chem. Res. 2007, 46, 5420–5428. [Google Scholar] [CrossRef]
- Chaudry, M.A.; Ahmad, S.; Malik, M.T. Supported liquid membrane technique applicability for removal of chromium from tannery wastes. Waste Manag. 1998, 17, 211–218. [Google Scholar] [CrossRef]
- Kislik, V.S. Carrier-Facilitated Coupled Transport Trough Liquid Membranes: General Theoretical Considerations and Influencing Parameters. In Liquid Membranes. Principles and Applications in Chemical Separations and Wastewater Treatment, 1st ed.; Kislik, V.S., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2010; pp. 17–71. [Google Scholar]














| Compound | Distillation Parameters | Yield (%) | Viscosity * (mPa s) | |
|---|---|---|---|---|
| Pressure (bar) (hPa) | Boiling Temp. (°C) | |||
| 1-oktylo-1,2,4-triazole | 1 | 185–188 | 78 | 6.834 |
| 1-nonylo-1,2,4-triazole | 1 | 201–203 | 70 | 8.464 |
| 1-decylo-1,2,4-triazole | 1 | 216–218 | 75 | 8.609 |
| 1-undecylo-1,2,4-triazole | 1 | 225–226 | 68 | 12.57 |
| 1-dodecylo-1,2,4-triazole | 1 | 239–241 | 73 | - |
| 1-tetradecylo-1,2,4-triazole | melting point = 47–49 °C | 66 | - | |
| Carrier | Content (wt %) | ||
|---|---|---|---|
| TRIA-n | ONPOE | CTA | |
| TRIA-8 | 11.3 | 65.2 | 23.5 |
| TRIA-9 | 12.1 | 64.6 | 23.3 |
| TRIA-10 | 12.9 | 64.0 | 23.1 |
| TRIA-11 | 13.6 | 63.5 | 22.9 |
| TRIA-12 | 14.3 | 63.0 | 22.7 |
| TRIA-14 | 15.1 | 62.4 | 22.5 |
| Module Diameter | 120 mm |
| Membrane diameter | 90 mm |
| Module thickness | 10 mm |
| Outer diameter of spiral | 77.0 mm |
| Channel depth | 0.25 mm |
| Channel width | 1.42 mm |
| Channel length | 239.2 cm |
| Effective channel area | 33.96 cm2 |
| Channel volume | 0.849 cm3 |
| Volumetric Flux (cm3/min) | Rate oAqueous Phase Exchange in 1 h | Contact Time (s) | Linear Speed (m/s) | Reynolds Number |
|---|---|---|---|---|
| 0.5 | 1.2 | 105.60 | 0.023 | 87 |
| 1.0 | 2.4 | 52.80 | 0.047 | 177 |
| 1.5 | 3.6 | 35.20 | 0.070 | 263 |
| 2.0 | 4.8 | 26.40 | 0.094 | 354 |
| 2.5 | 6.0 | 21.12 | 0.117 | 440 |
| 3.0 | 7.2 | 17.60 | 0.141 | 531 |
| 3.5 | 8.4 | 15.09 | 0.164 | 617 |
| 4.0 | 9.6 | 13.20 | 0.188 | 708 |
| 4.5 | 10.8 | 11.73 | 0.211 | 794 |
| Cl- (mol/dm3) | Recovery Coefficient, R (%) | |
|---|---|---|
| 6 h | 12 h | |
| 0.5 | 7.4 | 17.3 |
| 1.0 | 8.0 | 23.1 |
| 1.5 | 15.1 | 28.7 |
| 2.0 | 13.0 | 26.7 |
| 2.5 | 10.2 | 23.6 |
| 3.0 | 8.3 | 19.0 |
| 3.5 | 6.9 | 17.2 |
| 4.0 | 6.9 | 17.6 |
| 4.5 | 6.1 | 15.7 |
| 5.0 | 6.4 | 14.6 |
| Carrier | Recovery Coefficient, R (%) | |
|---|---|---|
| 6 h | 12 h | |
| TRIA-8 | 32.0 | 50.3 |
| TRIA-9 | 15.8 | 27.6 |
| TRIA-10 | 14.0 | 26.4 |
| TRIA-11 | 7.7 | 16.5 |
| TRIA-12 | 6.2 | 13.4 |
| TRIA-14 | 1.7 | 7.7 |
| Temperature (°C) | Recovery Coefficient, R (%) | |
|---|---|---|
| 6 h | 12 h | |
| 20 | 12.9 | 26.4 |
| 30 | 27.1 | 44.3 |
| 40 | 57.0 | 80.1 |
| 50 | 87.6 | 96.5 |
| Metal Ions | Carrier | |||||
|---|---|---|---|---|---|---|
| TRIA-8 | TRIA-10 | TRIA-12 | ||||
| J0 (μmol/(m2s)) | J0 (μmol/(m2s)) | J0 (μmol/(m2s)) | ||||
| Cu(II) | 17.3 ± 1.2 | . | 9.9 ± 0.5 | 1.90 ± 0.10 | ||
| Ni(II) | 0.376 ± 0.003 | 0.308 ± 0.008 | 0.0052 ± 0.0005 | |||
| Co(II) | 0.0304 ± 0.0017 | 0.049 ± 0.003 | 0.00215 ± 0.000 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajda, B.; Plackowski, R.; Skrzypczak, A.; Bogacki, M.B. Facilitated Transport of Copper(II) across Polymer Inclusion Membrane with Triazole Derivatives as Carrier. Membranes 2020, 10, 201. https://doi.org/10.3390/membranes10090201
Gajda B, Plackowski R, Skrzypczak A, Bogacki MB. Facilitated Transport of Copper(II) across Polymer Inclusion Membrane with Triazole Derivatives as Carrier. Membranes. 2020; 10(9):201. https://doi.org/10.3390/membranes10090201
Chicago/Turabian StyleGajda, Bernadeta, Radosław Plackowski, Andrzej Skrzypczak, and Mariusz B. Bogacki. 2020. "Facilitated Transport of Copper(II) across Polymer Inclusion Membrane with Triazole Derivatives as Carrier" Membranes 10, no. 9: 201. https://doi.org/10.3390/membranes10090201
APA StyleGajda, B., Plackowski, R., Skrzypczak, A., & Bogacki, M. B. (2020). Facilitated Transport of Copper(II) across Polymer Inclusion Membrane with Triazole Derivatives as Carrier. Membranes, 10(9), 201. https://doi.org/10.3390/membranes10090201