Optimization of Aquaporin Loading for Performance Enhancement of Aquaporin-Based Biomimetic Thin-Film Composite Membranes
Abstract
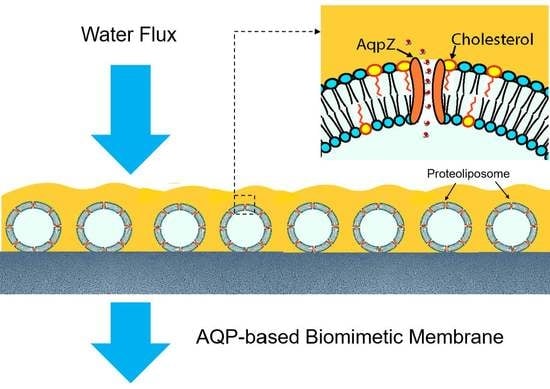
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.1.1. Chemicals for Proteoliposome Preparation and Characterization
2.1.2. Chemicals for Membrane Synthesis and Characterization
2.1.3. Expression and Purification of AqpZ and Mutant
2.2. Reconstitution of Proteoliposomes
2.3. Fabrication of ABMs
2.4. Characterizations of Proteoliposomes and ABMs
2.4.1. Size and Water Permeability of Liposomes and Proteoliposomes
2.4.2. Membrane Characterization
2.4.3. Evaluation of the Separation Performance of ABMs
3. Results and Discussion
3.1. Effect of Proteoliposome Concentration on the Characteristics and Separation Performance of ABMs
3.2. Effect of Protein-to-Lipid Ratio (PLR) on the Separation Performance of ABMs
3.3. Effect of Cholesterol on ABM Separation Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agre, P.; Sasaki, S.; Chrispeels, M.J. Aquaporins: A family of water channel proteins. Am. J. Physiol. Ren. Physiol. 1993, 265, F461. [Google Scholar] [CrossRef]
- Nielsen, S.; Agre, P. The aquaporin family of water channels in kidney. Kidney Int. 1995, 48, 1057–1068. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.; Frøkiær, J.; Marples, D.; Kwon, T.-H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: From molecules to medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Y.; Wang, R.; Hélix-Nielsen, C.; Fane, A. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Borgnia, M.J.; Kozono, D.; Calamita, G.; Maloney, P.C.; Agre, P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein11 edited by W. Baumeister. J. Mol. Biol. 1999, 291, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montemagno, C. Nanofabricated Membrane Using Polymerized Proteoliposomes. U.S. Patent US20120043275A1, 23 February 2012. [Google Scholar]
- Ibragimova, S.; Stibius, K.; Szewczykowski, P.; Perry, M.; Bohr, H.; Hélix-Nielsen, C. Hydrogels for in situ encapsulation of biomimetic membrane arrays. Polym. Adv. Technol. 2012, 23, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Perry, M.; Rein, C.; Vogel, J. Large scale biomimetic membrane arrays. In Biomimetic Membranes for Sensor and Separation Applications; Hélix-Nielsen, C., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 205–231. [Google Scholar]
- Lander, M.R.; Ibragimova, S.; Rein, C.; Vogel, J.; Stibius, K.; Geschke, O.; Perry, M.; Hélix-Nielsen, C. Biomimetic membrane arrays on cast hydrogel supports. Langmuir 2011, 27, 7002–7007. [Google Scholar] [CrossRef]
- Rein, C.; Pszon-Bartosz, K.; Stibius, K.B.; Bjørnholm, T.; Hélix-Nielsen, C. Free-standing biomimetic polymer membrane imaged with atomic force microscopy. Langmuir 2010, 27, 499–503. [Google Scholar] [CrossRef]
- Nielsen, C. Biomimetic membranes for sensor and separation applications. Anal. Bioanal. Chem. 2009, 395, 697–718. [Google Scholar] [CrossRef]
- Vogel, J.; Perry, M.; Hansen, J.S.; Bolinger, P.Y.; Nielsen, C.H.; Geschke, O. A support structure for biomimetic applications. J. Micromech. Microeng. 2009, 19, 025026. [Google Scholar] [CrossRef]
- Knoll, W.; Bender, K.; Förch, R.; Frank, C.; Götz, H.; Heibel, C.; Jenkins, T.; Jonas, U.; Kibrom, A.; Kügler, R.; et al. Polymer-tethered bimolecular lipid membranes. In Polymer Membranes/Biomembranes; Meier, W.P., Knoll, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 197–233. [Google Scholar]
- He, Y.; Hoi, H.; Montemagno, C.D.; Abraham, S. Functionalized polymeric membrane with aquaporin using click chemistry for water purification application. J. Appl. Polym. Sci. 2018, 135, 46678. [Google Scholar] [CrossRef]
- Sun, G.; Chung, T.-S.; Jeyaseelan, K.; Armugam, A. Stabilization and immobilization of aquaporin reconstituted lipid vesicles for water purification. Colloids Surf. B Biointerfaces 2013, 102, 466–471. [Google Scholar] [CrossRef]
- Wang, H.; Chung, T.-S.; Tong, Y.W.; Jeyaseelan, K.; Armugam, A.; Chen, Z.; Hong, M.; Meier, W. Highly permeable and selective pore-spanning biomimetic membrane embedded with aquaporin Z. Small 2012, 8, 1185–1190. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Wang, X.; Wang, S.; Ding, W.; Gao, C. Layer-by-layer assembly of aquaporin Z-incorporated biomimetic membranes for water purification. Environ. Sci. Technol. 2015, 49, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.S.; Chung, T.-S.; Jeyaseelan, K.; Armugam, A. Aquaporin-embedded biomimetic membranes for nanofiltration. J. Membr. Sci. 2012, 407–408, 27–33. [Google Scholar] [CrossRef]
- Xie, W.; He, F.; Wang, B.; Chung, T.-S.; Jeyaseelan, K.; Armugam, A.; Tong, Y.W. An aquaporin-based vesicle-embedded polymeric membrane for low energy water filtration. J. Mater. Chem. A 2013, 1, 7592–7600. [Google Scholar] [CrossRef]
- Kaufman, Y.; Grinberg, S.; Linder, C.; Heldman, E.; Gilron, J.; Freger, V. Fusion of bolaamphiphile micelles: A method to prepare stable supported biomimetic membranes. Langmuir 2013, 29, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, R.; Tang, C.; Vararattanavech, A.; Zhao, Y.; Torres, J.; Fane, T. Preparation of supported lipid membranes for aquaporin Z incorporation. Colloids Surf. B Biointerfaces 2012, 94, 333–340. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, C.; Li, X.; Vararattanavech, A.; Shen, W.; Torres, J.; Hélix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G. Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and RO performance characterization. J. Membr. Sci. 2012, 423–424, 422–428. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Wicaksana, F.; Tang, C.; Torres, J.; Fane, A.G. Preparation of high performance nanofiltration, NF, membranes incorporated with aquaporin Z. J. Membr. Sci. 2014, 450, 181–188. [Google Scholar] [CrossRef]
- Qi, S.; Wang, R.; Chaitra, G.K.M.; Torres, J.; Hu, X.; Fane, A.G. Aquaporin-based biomimetic reverse osmosis membranes: Stability and long term performance. J. Membr. Sci. 2016, 508, 94–103. [Google Scholar] [CrossRef]
- Hélix-Nielsen, C.; Zhao, Y.; Qiu, C.; Mentzel, S.; Torres, J.; Chuyang, T.; Geschke, O.; Fane, A.G. Robust high performance aquaporin based biomimetic membranes. Biophys. J. 2013, 104, 687a. [Google Scholar] [CrossRef] [Green Version]
- Fuwad, A.; Ryu, H.; Malmstadt, N.; Kim, S.M.; Jeon, T.-J. Biomimetic membranes as potential tools for water purification: Preceding and future avenues. Desalination 2019, 458, 97–115. [Google Scholar] [CrossRef]
- Çalıcıoğlu, N.G.; Özdemir, G.Ö.; Öztürk, A.; Yıldız, A.; Yılmaz, H.; Ergenekon, P.; Erbakan, M.; Erhan, E.; Özkan, M. Use of halophilic aquaporin for preparation of biomimetic thin film composite membrane. J. Membr. Sci. 2018, 568, 105–112. [Google Scholar] [CrossRef]
- Sengur-Tasdemir, R.; Sayinli, B.; Urper, G.M.; Tutuncu, H.E.; Gul-Karaguler, N.; Ates-Genceli, E.; Tarabara, V.V.; Koyuncu, I. Hollow fiber nanofiltration membranes with integrated aquaporin Z. New J. Chem. 2018, 42, 17769–17778. [Google Scholar] [CrossRef]
- Engelhardt, S.; Sadek, A.; Duirk, S. Rejection of trace organic water contaminants by an Aquaporin-based biomimetic hollow fiber membrane. Sep. Purif. Technol. 2018, 197, 170–177. [Google Scholar] [CrossRef]
- Mirshekar, L.; Kamarehie, B.; Jafari, A.; Ghaderpoori, M.; Karami, M.A.; Sahebi, S. Performance evaluation of aquaporin forward osmosis membrane using chemical fertilizers as a draw solution. Environ. Prog. Sustain. Energy 2021, 40, e13536. [Google Scholar] [CrossRef]
- Salamanca, M.; López-Serna, R.; Palacio, L.; Hernández, A.; Prádanos, P.; Peña, M. Study of the rejection of contaminants of emerging concern by a biomimetic aquaporin hollow fiber forward osmosis membrane. J. Water Process. Eng. 2021, 40, 101914. [Google Scholar] [CrossRef]
- Li, R.; Braekevelt, S.; de Carfort, J.L.N.; Hussain, S.; Bollmann, U.E.; Bester, K. Laboratory and pilot evaluation of aquaporin-based forward osmosis membranes for rejection of micropollutants. Water Res. 2021, 194, 116924. [Google Scholar] [CrossRef]
- Valverde-Pérez, B.; Pape, M.L.; Kjeldgaard, A.F.; Zachariae, A.A.; Schneider, C.; Hélix-Nielsen, C.; Zarebska, A.; Smets, B.F. Dewatering methanotrophic enrichments intended for single cell protein production using biomimetic aquaporin forward osmosis membranes. Sep. Purif. Technol. 2020, 235, 116133. [Google Scholar] [CrossRef]
- Chun, Y.; Qing, L.; Sun, G.; Bilad, M.R.; Fane, A.G.; Chong, T.H. Prototype aquaporin-based forward osmosis membrane: Filtration properties and fouling resistance. Desalination 2018, 445, 75–84. [Google Scholar] [CrossRef]
- Singh, N.; Petrinic, I.; Hélix-Nielsen, C.; Basu, S.; Balakrishnan, M. Concentrating molasses distillery wastewater using biomimetic forward osmosis, FO, membranes. Water Res. 2018, 130, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Luo, W.; Guo, H.; Nghiem, L.D.; Tang, C.Y.; Gray, S.R. Trace organic contaminant rejection by aquaporin forward osmosis membrane: Transport mechanisms and membrane stability. Water Res. 2018, 132, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Hélix-Nielsen, C.; Su, H.; Torres, J.; Tang, C.; Fane, R.W.A.; Mu, Y. Population shift between the open and closed states changes the water permeability of an aquaporin Z mutant. Biophys. J. 2012, 103, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castile, J.D.; Taylor, K.M. Factors affecting the size distribution of liposomes produced by freeze–thaw extrusion. Int. J. Pharm. 1999, 188, 87–95. [Google Scholar] [CrossRef]
- Hansen, J.S.; Vararattanavech, A.; Plasencia, I.P.G., Jr.; Bomholt, J.; Torres, J.; Emnéus, J.; Hélix-Nielsen, C. Interaction between sodium dodecyl sulfate and membrane reconstituted aquaporins: A comparative study of spinach SoPIP2; 1 and E. coli AqpZ. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2600–2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Ning, L.; Tan, C.-H.; Mu, Y.; Wang, R. Hyperfast water transport through biomimetic nanochannels from peptide-attached, pR-pillar[5]arene. Small 2019, 15, 1804678. [Google Scholar] [CrossRef]
- Zhao, Y.; Vararattanavech, A.; Li, X.; HélixNielsen, C.; Vissing, T.; Torres, J.; Wang, R.; Fane, A.G.; Tang, C.Y. Effects of proteoliposome composition and draw solution types on separation performance of aquaporin-based proteoliposomes: Implications for seawater desalination using aquaporin-based biomimetic membranes. Environ. Sci. Technol. 2013, 47, 1496–1503. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Wicaksana, F.; Zhao, Y.; Tang, C.; Torres, J.; Fane, A.G. Fusion behaviour of aquaporin Z incorporated proteoliposomes investigated by quartz crystal microbalance with dissipation, QCM-D). Colloids Surf. B Biointerfaces 2013, 111, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chou, S.; Wang, R.; Shi, L.; Fang, W.; Chaitra, G.; Tang, C.Y.; Torres, J.; Hu, X.; Fane, A.G. Nature gives the best solution for desalination: Aquaporin-based hollow fiber composite membrane with superior performance. J. Membr. Sci. 2015, 494, 68–77. [Google Scholar] [CrossRef]
- Li, X.; Loh, C.H.; Wang, R.; Widjajanti, W.; Torres, J. Fabrication of a robust high-performance FO membrane by optimizing substrate structure and incorporating aquaporin into selective layer. J. Membr. Sci. 2017, 525, 257–268. [Google Scholar] [CrossRef]
- Tong, J.; Briggs, M.M.; McIntosh, T.J. Water permeability of aquaporin-4 channel depends on bilayer composition, thickness, and elasticity. Biophys. J. 2012, 103, 1899–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driessen, A.J.M.; van de Vossenberg, J.L.C.M.; Konings, W.N. Membrane composition and ion-permeability in extremophiles. FEMS Microbiol. Rev. 1996, 18, 139–148. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, X.; Wei, J.; Torres, J.; Fane, A.G.; Wang, R.; Tang, C.Y. Optimization of Aquaporin Loading for Performance Enhancement of Aquaporin-Based Biomimetic Thin-Film Composite Membranes. Membranes 2022, 12, 32. https://doi.org/10.3390/membranes12010032
Zhao Y, Li X, Wei J, Torres J, Fane AG, Wang R, Tang CY. Optimization of Aquaporin Loading for Performance Enhancement of Aquaporin-Based Biomimetic Thin-Film Composite Membranes. Membranes. 2022; 12(1):32. https://doi.org/10.3390/membranes12010032
Chicago/Turabian StyleZhao, Yang, Xuesong Li, Jing Wei, Jaume Torres, Anthony G. Fane, Rong Wang, and Chuyang Y. Tang. 2022. "Optimization of Aquaporin Loading for Performance Enhancement of Aquaporin-Based Biomimetic Thin-Film Composite Membranes" Membranes 12, no. 1: 32. https://doi.org/10.3390/membranes12010032
APA StyleZhao, Y., Li, X., Wei, J., Torres, J., Fane, A. G., Wang, R., & Tang, C. Y. (2022). Optimization of Aquaporin Loading for Performance Enhancement of Aquaporin-Based Biomimetic Thin-Film Composite Membranes. Membranes, 12(1), 32. https://doi.org/10.3390/membranes12010032