Efficient Anion-Exchange Membranes with Anti-Scaling Properties Obtained by Surface Modification of Commercial Membranes Using a Polyquaternium-22
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes
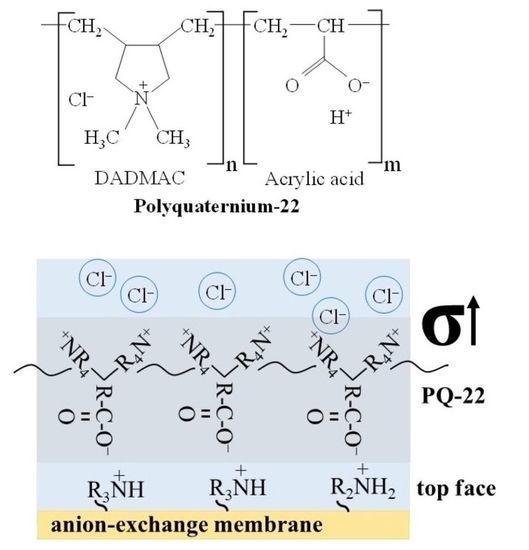
2.2. Methods
2.3. Flow-Through Electrodialysis Cell for Measuring Electrochemical Characteristics
2.4. Streaming and Zeta Potential of Membrane Surface
2.5. Visualization of Electroconvective Vortices
3. Results and Discussion
3.1. Electrochemical Characteristics of Pristine and Modified Membranes
3.2. Analysis of the Visualization of the Formation of Electroconvective Vortices
3.3. Evaluation of Scale Resistance of Anion-Exchange Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Processing of Current-Voltage Curves
Appendix A.2. Processing of Electrochemical Impedance Spectra

References
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- El Rayess, Y.; Mietton-Peuchot, M. Membrane Technologies in Wine Industry: An Overview. Crit. Rev. Food Sci. Nutr. 2016, 56, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, C. Electrodialysis-based separation technologies: A critical review. AIChE J. 2008, 54, 3147–3159. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Kikhavani, T.; Ashrafizadeh, S.N.; Van der Bruggen, B. Identification of optimum synthesis conditions for a novel anion exchange membrane by response surface methodology. J. Appl. Polym. Sci. 2014, 131, 39888. [Google Scholar] [CrossRef]
- Marcos-Madrazo, A.; Casado-Coterillo, C.; García-Cruz, L.; Iniesta, J.; Simonelli, L.; Sebastián, V.; Encabo-Berzosa, M.; Arruebo, M.; Irabien, Á. Preparation and Identification of Optimal Synthesis Conditions for a Novel Alkaline Anion-Exchange Membrane. Polymers 2018, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Guo, J.; Zhou, J.; Xu, Q.; Chu, D.; Chen, R. Novel nanostructured high-performance anion exchange ionomers for anion exchange membrane fuel cells. J. Power Sources 2012, 202, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Highly Hydrophilic Thin-Film Composite Forward Osmosis Membranes Functionalized with Surface-Tailored Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 5044–5053. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Memb. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Pan, Q.; Hossain, M.M.; Yang, Z.; Wang, Y.; Wu, L.; Xu, T. One-pot solvent-free synthesis of cross-linked anion exchange membranes for electrodialysis. J. Memb. Sci. 2016, 515, 115–124. [Google Scholar] [CrossRef]
- Tanaka, Y.; Senō, M. Treatment of ion exchange membranes to decrease divalent ion permeability. J. Memb. Sci. 1981, 8, 115–127. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Memb. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.; Miao, J.; Xu, T. Monovalent cation perm-selective membranes (MCPMs): New developments and perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Memb. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Abdu, S.; Martí-Calatayud, M.-C.; Wong, J.E.; García-Gabaldón, M.; Wessling, M. Layer-by-Layer Modification of Cation Exchange Membranes Controls Ion Selectivity and Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1843–1854. [Google Scholar] [CrossRef]
- Porada, S.; van Egmond, W.J.; Post, J.W.; Saakes, M.; Hamelers, H.V.M. Tailoring ion exchange membranes to enable low osmotic water transport and energy efficient electrodialysis. J. Memb. Sci. 2018, 552, 22–30. [Google Scholar] [CrossRef]
- Melnikov, S.; Shkirskaya, S. Transport properties of bilayer and multilayer surface-modified ion-exchange membranes. J. Memb. Sci. 2019, 590, 117272. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells—Fundamentals and Applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kim, N.H.; Jung, D.; Lee, J.H. Enhanced mechanical properties and proton conductivity of Nafion–SPEEK–GO composite membranes for fuel cell applications. J. Memb. Sci. 2014, 458, 128–135. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Q.; Wu, H.; Deng, X.; Yang, Q.; Liu, P.; Hong, Y.; Zhu, A.; Liu, Q. Dual hydrophobic modifications toward anion exchange membranes with both high ion conductivity and excellent dimensional stability. J. Memb. Sci. 2020, 595, 117521. [Google Scholar] [CrossRef]
- Zabolotsky, V.; Utin, S.; Bespalov, A.; Strelkov, V. Modification of asymmetric bipolar membranes by functionalized hyperbranched polymers and their investigation during pH correction of diluted electrolytes solutions by electrodialysis. J. Memb. Sci. 2015, 494, 188–195. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Belova, E.I.; Nikonenko, V.V.; Zabolotsky, V.I.; Lopatkova, G.Y.; Karzhavin, Y.N.; Larchet, C. Lower rate of H+ (OH−) ions generation at an anion-exchange membrane in electrodialysis. Desalin. Water Treat. 2010, 21, 109–114. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Akberova, E.M.; Zabolotskii, V.I. Electroconvection in systems with heterogeneous ion-exchange membranes after thermal modification. Russ. J. Electrochem. 2017, 53, 398–410. [Google Scholar] [CrossRef]
- Kang, M.-S.; Choi, Y.-J.; Lee, H.-J.; Moon, S.-H. Effects of inorganic substances on water splitting in ion-exchange membranes. J. Colloid Interface Sci. 2004, 273, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Zabolotsky, V.I.; Novak, L.; Kovalenko, A.V.; Nikonenko, V.V.; Urtenov, M.H.; Lebedev, K.A.; But, A.Y. Electroconvection in systems with heterogeneous ion-exchange membranes. Pet. Chem. 2017, 57, 779–789. [Google Scholar] [CrossRef]
- Pawlowski, S.; Crespo, J.G.; Velizarov, S. Profiled ion exchange membranes: A comprehensible review. Int. J. Mol. Sci. 2019, 20, 165. [Google Scholar] [CrossRef] [Green Version]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Gurreri, L.; Filingeri, A.; Ciofalo, M.; Cipollina, A.; Tedesco, M.; Tamburini, A.; Micale, G. Electrodialysis with asymmetrically profiled membranes: Influence of profiles geometry on desalination performance and limiting current phenomena. Desalination 2021, 506, 115001. [Google Scholar] [CrossRef]
- Jiang, W.; Lin, L.; Xu, X.; Wang, H.; Xu, P. Physicochemical and electrochemical characterization of cation-exchange membranes modified with polyethyleneimine for elucidating enhanced monovalent permselectivity of electrodialysis. J. Memb. Sci. 2019, 572, 545–556. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Yaroshchuk, A.; Bruening, M.L. Coating of Nafion Membranes with Polyelectrolyte Multilayers to Achieve High Monovalent/Divalent Cation Electrodialysis Selectivities. ACS Appl. Mater. Interfaces 2015, 7, 6620–6628. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Surface modification of an anion exchange membrane to improve the selectivity for monovalent anions in electrodialysis—Experimental verification of theoretical predictions. J. Memb. Sci. 2015, 490, 301–310. [Google Scholar] [CrossRef]
- Butylskii, D.Y.; Troitskiy, V.A.; Skudarnova, A.S.; Sharafan, M.V.; Pismenskaya, N.D. Stability of Properties of a Modified Anion-Exchange Membrane Obtained by Treating the Surface of a Commercial Sample with Bifunctional Polymer Containing Quaternary Amino Groups. Membr. Membr. Technol. 2021, 3, 291–301. [Google Scholar] [CrossRef]
- Balster, J.; Krupenko, O.; Punt, I.; Stamatialis, D.; Wessling, M. Preparation and characterisation of monovalent ion selective cation exchange membranes based on sulphonated poly(ether ether ketone). J. Memb. Sci. 2005, 263, 137–145. [Google Scholar] [CrossRef]
- Kerres, J.; Cui, W.; Disson, R.; Neubrand, W. Development and characterization of crosslinked ionomer membranes based upon sulfinated and sulfonated PSU crosslinked PSU blend membranes by disproportionation of sulfinic acid groups. J. Memb. Sci. 1998, 139, 211–225. [Google Scholar] [CrossRef]
- Ali, I.; Raza, M.A.; Mehmood, R.; Islam, A.; Sabir, A.; Gull, N.; Haider, B.; Park, S.H.; Khan, R.U. Novel Maleic Acid, Crosslinked, Nanofibrous Chitosan/Poly (Vinylpyrrolidone) Membranes for Reverse Osmosis Desalination. Int. J. Mol. Sci. 2020, 21, 7338. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Liu, H.; Van der Bruggen, B.; Sotto Díaz, A.; Shen, J.; Gao, C. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity. J. Memb. Sci. 2016, 520, 262–271. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Zhuang, L.; Ding, L.-X.; Wang, H. Introduction of metal precursors by electrodeposition for the in situ growth of metal–organic framework membranes on porous metal substrates. J. Mater. Chem. A 2017, 5, 1948–1951. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Kumar, M.; Rajesh, K.P.; Shahi, V.K.; Natarajan, T.S. Nano-fibrous sulfonated poly(ether ether ketone) membrane for selective electro-transport of ions. Sep. Purif. Technol. 2010, 75, 174–182. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Liu, F.; Ding, S.; Wang, X.; Du, G.; Liu, J.; Apel, P.; Kluth, P.; Trautmann, C.; et al. Ultrafast ion sieving using nanoporous polymeric membranes. Nat. Commun. 2018, 9, 569. [Google Scholar] [CrossRef]
- Sheng, F.; Afsar, N.U.; Zhu, Y.; Ge, L.; Xu, T. PVA-Based Mixed Matrix Membranes Comprising ZSM-5 for Cations Separation. Membranes 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic–inorganic composite membranes for fouling resistance improvement. J. Memb. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Mizutani, Y.; Yamane, R.; Ihara, H.; Motomura, H. Studies of Ion Exchange Membranes. XVI. The Preparation of Ion Exchange Membranes by the “Paste Method”. Bull. Chem. Soc. Jpn. 1963, 36, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Strathmann, H. Ion-Exchange Membranes. In Membrane Handbook; Springer US: Boston, MA, USA, 1992; pp. 230–245. [Google Scholar]
- Sata, T. Ion Exchange Membranes: Preparation, Characterization, Modification and Application; Royal Society of Chemistry: Cambridge, UK, 2007; ISBN 978-0-85404-590-7. [Google Scholar]
- Rubinstein, I.; Maletzki, F. Electroconvection at an electrically inhomogeneous permselective membrane surface. J. Chem. Soc. Faraday Trans. 1991, 87, 2079–2087. [Google Scholar] [CrossRef]
- Mishchuk, N.A. Electro-osmosis of the second kind near the heterogeneous ion-exchange membrane. Colloids Surf. A Physicochem. Eng. Asp. 1998, 140, 75–89. [Google Scholar] [CrossRef]
- Mishchuk, N.A. Polarization of systems with complex geometry. Curr. Opin. Colloid Interface Sci. 2013, 18, 137–148. [Google Scholar] [CrossRef]
- Davidson, S.M.; Wessling, M.; Mani, A. On the Dynamical Regimes of Pattern-Accelerated Electroconvection. Sci. Rep. 2016, 6, 22505. [Google Scholar] [CrossRef] [Green Version]
- Zyryanova, S.; Mareev, S.; Gil, V.; Korzhova, E.; Pismenskaya, N.; Sarapulova, V.; Rybalkina, O.; Boyko, E.; Larchet, C.; Dammak, L.; et al. How Electrical Heterogeneity Parameters of Ion-Exchange Membrane Surface Affect the Mass Transfer and Water Splitting Rate in Electrodialysis. Int. J. Mol. Sci. 2020, 21, 973. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, I.; Zaltzman, B.; Pundik, T. Ion-exchange funneling in thin-film coating modification of heterogeneous electrodialysis membranes. Phys. Rev. E 2002, 65, 041507. [Google Scholar] [CrossRef]
- Choi, J.-H.; Moon, S.-H. Pore size characterization of cation-exchange membranes by chronopotentiometry using homologous amine ions. J. Memb. Sci. 2001, 191, 225–236. [Google Scholar] [CrossRef]
- Ward, K.R.; Lawrence, N.S.; Hartshorne, R.S.; Compton, R.G. The theory of cyclic voltammetry of electrochemically heterogeneous surfaces: Comparison of different models for surface geometry and applications to highly ordered pyrolytic graphite. Phys. Chem. Chem. Phys. 2012, 14, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Butylskii, D.Y.; Mareev, S.A.; Pismenskaya, N.D.; Apel, P.Y.; Polezhaeva, O.A.; Nikonenko, V.V. Phenomenon of two transition times in chronopotentiometry of electrically inhomogeneous ion exchange membranes. Electrochim. Acta 2018, 273, 289–299. [Google Scholar] [CrossRef]
- Mareev, S.; Kozmai, A.; Nikonenko, V.; Belashova, E.; Pourcelly, G.; Sistat, P. Chronopotentiometry and impedancemetry of homogeneous and heterogeneous ion-exchange membranes. Desalin. Water Treat. 2014, 56, 3207–3210. [Google Scholar] [CrossRef]
- Dukhin, S.S. Electrokinetic phenomena of the second kind and their applications. Adv. Colloid Interface Sci. 1991, 35, 173–196. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Equilibrium Electroconvective Instability. Phys. Rev. Lett. 2015, 114, 114502. [Google Scholar] [CrossRef] [Green Version]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electroconvective instability. J. Fluid Mech. 2007, 579, 173–226. [Google Scholar] [CrossRef]
- Nebavskaya, K.A.; Sarapulova, V.V.; Sabbatovskiy, K.G.; Sobolev, V.D.; Pismenskaya, N.D.; Sistat, P.; Cretin, M.; Nikonenko, V.V. Impact of ion exchange membrane surface charge and hydrophobicity on electroconvection at underlimiting and overlimiting currents. J. Memb. Sci. 2017, 523, 36–44. [Google Scholar] [CrossRef]
- Barragán, V.M.; Villaluenga, J.P.G.; Izquierdo-Gil, M.A.; Kristiansen, K.R. On the electrokinetic characterization of charged polymeric membranes by transversal streaming potential. Electrochim. Acta 2021, 387, 138462. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Wang, P.; Wang, Z.; Shi, F.; Liu, H. Investigations on the interfacial capacitance and the diffusion boundary layer thickness of ion exchange membrane using electrochemical impedance spectroscopy. J. Memb. Sci. 2016, 502, 37–47. [Google Scholar] [CrossRef]
- Somovilla, P.; Villaluenga, J.P.G.; Barragán, V.M.; Izquierdo-Gil, M.A. Experimental determination of the streaming potential across cation-exchange membranes with different morphologies. J. Memb. Sci. 2016, 500, 16–24. [Google Scholar] [CrossRef]
- Andersen, M.B.; van Soestbergen, M.; Mani, A.; Bruus, H.; Biesheuvel, P.M.; Bazant, M.Z. Current-Induced Membrane Discharge. Phys. Rev. Lett. 2012, 109, 108301. [Google Scholar] [CrossRef] [PubMed]
- Pismenskaya, N.D.; Pokhidnia, E.V.; Pourcelly, G.; Nikonenko, V.V. Can the electrochemical performance of heterogeneous ion-exchange membranes be better than that of homogeneous membranes? J. Memb. Sci. 2018, 566, 54–68. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Mareev, S.A.; Pokhidnya, E.V.; Larchet, C.; Dammak, L.; Nikonenko, V.V. Effect of Surface Modification of Heterogeneous Anion-Exchange Membranes on the Intensity of Electroconvection at Their Surfaces. Russ. J. Electrochem. 2019, 55, 1203–1220. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Bondarev, D.A.; Bespalov, A.V. Electrochemical and Mass Transport Characteristics of the Strongly Basic MA-41 Membrane Modified by Poly-N,N-Diallylmorpholinium. Russ. J. Electrochem. 2018, 54, 963–969. [Google Scholar] [CrossRef]
- Xiao, X.; Shehzad, M.A.; Yasmin, A.; Ge, Z.; Liang, X.; Sheng, F.; Ji, W.; Ge, X.; Wu, L.; Xu, T. Anion permselective membranes with chemically-bound carboxylic polymer layer for fast anion separation. J. Memb. Sci. 2020, 614, 118553. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Lin, J.; Ye, W.; Xu, Y.; Shen, J.; Gao, C.; Bruggen, B. Van der Mono-valent cation selective membranes for electrodialysis by introducing polyquaternium-7 in a commercial cation exchange membrane. J. Memb. Sci. 2015, 486, 89–96. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, S.; Cao, H.; Li, Y.; Van der Bruggen, B. Layer-by-layer assembly of anion exchange membrane by electrodeposition of polyelectrolytes for improved antifouling performance. J. Memb. Sci. 2018, 558, 1–8. [Google Scholar] [CrossRef]
- Hong, J.G.; Park, T.-W. Electrochemical characterizations and reverse electrodialysis performance of hybrid anion exchange membranes for salinity gradient energy. J. Electroanal. Chem. 2018, 817, 134–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Lang, Q.; Tan, M.; Zhang, Y. Composite anion exchange membrane made by layer-by-layer method for selective ion separation and water migration control. Sep. Purif. Technol. 2018, 192, 278–286. [Google Scholar] [CrossRef]
- Arı, G.A.; Özcan, Z. A novel approach for stable anion exchange membrane: Self-assembled multilayer formation on the membrane via LbL method. Synth. Met. 2016, 220, 269–275. [Google Scholar] [CrossRef]
- Website of MEGA, a.s. Available online: https://www.mega.cz/membranes/ (accessed on 15 September 2022).
- Bulejko, P.; Stránská, E.; Weinertová, K. Electrochemical and mechanical stability of ion-exchange membranes in alkaline solution. Chem. Pap. 2017, 71, 1303–1309. [Google Scholar] [CrossRef]
- Slavinskaya, G.V.; Kurenkova, O.V. On the multifunctional character of strong basic anion-exchange resin. Sorpt. Chromatogr. Process. 2019, 19, 101–110. [Google Scholar] [CrossRef]
- Sabbatovskii, K.G.; Vilenskii, A.I.; Sobolev, V.D. Electrosurface properties of poly(ethylene terephthalate) films irradiated by heavy ions and track membranes based on these films. Colloid J. 2016, 78, 573–575. [Google Scholar] [CrossRef]
- Yaroshchuk, A.; Luxbacher, T. Interpretation of Electrokinetic Measurements with Porous Films: Role of Electric Conductance and Streaming Current within Porous Structure. Langmuir 2010, 26, 10882–10889. [Google Scholar] [CrossRef] [PubMed]
- Sedkaoui, Y.; Szymczyk, A.; Lounici, H.; Arous, O. A new lateral method for characterizing the electrical conductivity of ion-exchange membranes. J. Memb. Sci. 2016, 507, 34–42. [Google Scholar] [CrossRef]
- Hagmeyer, G.; Gimbel, R. Modelling the salt rejection of nanofiltration membranes for ternary ion mixturs and for single salts at different pH values. Desalination 1998, 117, 247–256. [Google Scholar] [CrossRef]
- Afonso, M.D.; Hagmeyer, G.; Gimbel, R. Streaming potential measurements to assess the variation of nanofiltration membranes surface charge with the concentration of salt solutions. Sep. Purif. Technol. 2001, 22–23, 529–541. [Google Scholar] [CrossRef]
- Lukáš, J.; Richau, K.; Schwarz, H.-H.; Paul, D. Surface characterization of polyelectrolyte complex membranes based on sodium cellulose sulfate and various cationic components. J. Memb. Sci. 1997, 131, 39–47. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, H.-J.; Choi, S.-J.; Geckeler, K.E.; Cho, J.; Moon, S.-H. Fouling mitigation of anion exchange membrane by zeta potential control. J. Colloid Interface Sci. 2003, 259, 293–300. [Google Scholar] [CrossRef]
- Butt, H.-J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Magut, P.K.S.; Das, S.; Fernand, V.E.; Losso, J.; McDonough, K.; Naylor, B.M.; Aggarwal, S.; Warner, I.M. Tunable Cytotoxicity of Rhodamine 6G via Anion Variations. J. Am. Chem. Soc. 2013, 135, 15873–15879. [Google Scholar] [CrossRef]
- Kwak, R.; Guan, G.; Peng, W.K.; Han, J. Microscale electrodialysis: Concentration profiling and vortex visualization. Desalination 2013, 308, 138–146. [Google Scholar] [CrossRef]
- Mishchuk, N.A. Concentration polarization of interface and non-linear electrokinetic phenomena. Adv. Colloid Interface Sci. 2010, 160, 16–39. [Google Scholar] [CrossRef]
- Belloň, T.; Polezhaev, P.; Vobecká, L.; Slouka, Z. Fouling of a heterogeneous anion-exchange membrane and single anion-exchange resin particle by ssDNA manifests differently. J. Memb. Sci. 2019, 572, 619–631. [Google Scholar] [CrossRef]
- Belloň, T.; Polezhaev, P.; Vobecká, L.; Svoboda, M.; Slouka, Z. Experimental observation of phenomena developing on ion-exchange systems during current-voltage curve measurement. J. Memb. Sci. 2019, 572, 607–618. [Google Scholar] [CrossRef]
- Slouka, Z.; Senapati, S.; Yan, Y.; Chang, H.-C. Charge Inversion, Water Splitting, and Vortex Suppression Due to DNA Sorption on Ion-Selective Membranes and Their Ion-Current Signatures. Langmuir 2013, 29, 8275–8283. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Zhil’tsova, A.V.; Malykhin, M.D.; Zabolotskii, V.I.; Lebedev, K.A.; Chermit, R.K.; Sharafan, M.V. Effect of the chemical nature of the ionogenic groups of ion-exchange membranes on the size of the electroconvective instability region in high-current modes. Russ. J. Electrochem. 2014, 50, 120–128. [Google Scholar] [CrossRef]
- Kang, M.-S.; Choi, Y.-J.; Moon, S.-H. Effects of charge density on water splitting at cation-exchange membrane surface in the over-limiting current region. Korean J. Chem. Eng. 2004, 21, 221–229. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Shel’deshov, N.V.; Gnusin, N.P. Dissociation of Water Molecules in Systems with Ion-exchange Membranes. Russ. Chem. Rev. 1988, 57, 801–808. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Sharafan, M.V.; Shel’deshov, N.V. Influence of the nature of membrane ionogenic groups on water dissociation and electrolyte ion transport: A rotating membrane disk study. Russ. J. Electrochem. 2008, 44, 1127–1134. [Google Scholar] [CrossRef]
- Simons, R. Water splitting in ion exchange membranes. Electrochim. Acta 1985, 30, 275–282. [Google Scholar] [CrossRef]
- Butylskii, D.Y.; Troitskiy, V.A.; Sharafan, M.V.; Pismenskaya, N.D.; Nikonenko, V.V. Scaling-resistant anion-exchange membrane prepared by in situ modification with a bifunctional polymer containing quaternary amino groups. Desalination 2022, 537, 115821. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; Barsoukov, E., Macdonald, J.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 9780471716242. [Google Scholar]
- Moya, A.A. A ladder network modelling the electrochemical impedance of the diffusion and reaction processes in semi-infinite space. Phys. Chem. Chem. Phys. 2016, 18, 3812–3816. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.A. Influence of dc electric current on the electrochemical impedance of ion-exchange membrane systems. Electrochim. Acta 2011, 56, 3015–3022. [Google Scholar] [CrossRef]
- Grafov, B.M.; Ukshe, E.A. Electrochemical AC Circuits; Nauka: Moscow, Russia, 1973. [Google Scholar]
- Beley, I.I.; Karmatskikh, S.A.; Rechapov, D.A.; Tsypkin, E.B.; Korostelev, A.S.; Antonenko, D.V. The results of the study of cement slurry stone corrosion resistance during interaction with high-mineralized formation waters of the Eastern Siberia deposits. Constr. Oil Gas Wells L. Sea 2018, 4, 23–30. [Google Scholar] [CrossRef]
- Butylskii, D.Y.; Troitskiy, V.A.; Skudarnova, A.S.; Sharafan, M. V Scaling on the Surface of an MA-41P Anion-Exchange Membrane in the Concentration Chamber of an Electrodialyzer during Processing of Dilute Stratal Water Imitates. Membr. Membr. Technol. 2022, 4, 336–346. [Google Scholar] [CrossRef]
- Gil, V.V.; Porozhnyy, M.V.; Rybalkina, O.A.; Sabbatovskiy, K.G.; Nikonenko, V.V. Modification of a heterogeneous cation-exchange membrane by Ti-Si based particles to enhance electroconvection and mitigate scaling during electrodialysis. Electrochim. Acta 2021, 391, 138913. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Tsygurina, K.A.; Sarapulova, V.V.; Mareev, S.A.; Nikonenko, V.V.; Pismenskaya, N.D. Evolution of Current–Voltage Characteristics and Surface Morphology of Homogeneous Anion-Exchange Membranes during the Electrodialysis Desalination of Alkali Metal Salt Solutions. Membr. Membr. Technol. 2019, 1, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Pine, S.H. The Base-Promoted Rearrangements of Quaternary Ammonium Salts. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1970; pp. 403–464. [Google Scholar]
- Gnusin, N.P.; Zabolotskii, V.I.; Nikonenko, V.V.; Urtenov, M.K. Convective-Diffusion Model of Electrodialytic Desalination. Limiting Current and Diffusion Layer. Sov. Electrochem. 1986, 22, 273–278. [Google Scholar]
- Rösler, H.-W.; Maletzki, F.; Staude, E. Ion transfer across electrodialysis membranes in the overlimiting current range: Chronopotentiometric studies. J. Memb. Sci. 1992, 72, 171–179. [Google Scholar] [CrossRef]
- Belova, E.I.; Lopatkova, G.Y.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Pourcelly, G. Effect of Anion-exchange Membrane Surface Properties on Mechanisms of Overlimiting Mass Transfer. J. Phys. Chem. B 2006, 110, 13458–13469. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Kozmai, A.E. Electrical equivalent circuit of an ion-exchange membrane system. Electrochim. Acta 2011, 56, 1262–1269. [Google Scholar] [CrossRef]












| Membranes | Ralex AHM-PES | MA-41P |
|---|---|---|
| Ion-exchange groups | –N+(R)3 | =NH; ≡N; –N+(R)3 |
| Thickness in 0.02 M NaCl, μm | 550 ± 3 | 510 ± 3 |
| Water content, gH2O/gdry, % | 46 ± 5 | 55 ± 2 |
| Exchange capacity, mmol g−1 wet | 1.33 ± 0.01 | 0.92 ± 0.05 |
| Conductivity in 0.5 M NaCl, κ, mS/cm | 6.7 | 10.5 |
| Sample | Zeta Potential, ζ, mV | Surface Charge, σ, µC cm−2 |
|---|---|---|
| MA-41P | 14.8 | 0.49 |
| MA-41Pmod | 20.3 | 0.69 |
| Ralex AHM-PES | 21.7 | 0.74 |
| Ralex AHM-PESmod | 33.4 | 1.18 |
| Sample | −Z”, Ohm | RΏ, Ohm | C, μF | |
|---|---|---|---|---|
| MA-41P | 28,189 | 37.6 | 60.3 | 5.6 |
| MA-41Pmod | 25,786 | 28.2 | 53.2 | 6.2 |
| Ralex AHM-PES | 22,182 | 26.0 | 55.2 | 7.2 |
| Ralex AHM-PESmod | 17,456 | 27.4 | 50.3 | 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butylskii, D.Y.; Troitskiy, V.A.; Ponomar, M.A.; Moroz, I.A.; Sabbatovskiy, K.G.; Sharafan, M.V. Efficient Anion-Exchange Membranes with Anti-Scaling Properties Obtained by Surface Modification of Commercial Membranes Using a Polyquaternium-22. Membranes 2022, 12, 1065. https://doi.org/10.3390/membranes12111065
Butylskii DY, Troitskiy VA, Ponomar MA, Moroz IA, Sabbatovskiy KG, Sharafan MV. Efficient Anion-Exchange Membranes with Anti-Scaling Properties Obtained by Surface Modification of Commercial Membranes Using a Polyquaternium-22. Membranes. 2022; 12(11):1065. https://doi.org/10.3390/membranes12111065
Chicago/Turabian StyleButylskii, Dmitrii Y., Vasiliy A. Troitskiy, Maria A. Ponomar, Ilya A. Moroz, Konstantin G. Sabbatovskiy, and Mikhail V. Sharafan. 2022. "Efficient Anion-Exchange Membranes with Anti-Scaling Properties Obtained by Surface Modification of Commercial Membranes Using a Polyquaternium-22" Membranes 12, no. 11: 1065. https://doi.org/10.3390/membranes12111065
APA StyleButylskii, D. Y., Troitskiy, V. A., Ponomar, M. A., Moroz, I. A., Sabbatovskiy, K. G., & Sharafan, M. V. (2022). Efficient Anion-Exchange Membranes with Anti-Scaling Properties Obtained by Surface Modification of Commercial Membranes Using a Polyquaternium-22. Membranes, 12(11), 1065. https://doi.org/10.3390/membranes12111065