Ionic Liquid-Mediated Interfacial Polymerization for Fabrication of Reverse Osmosis Membranes
Abstract
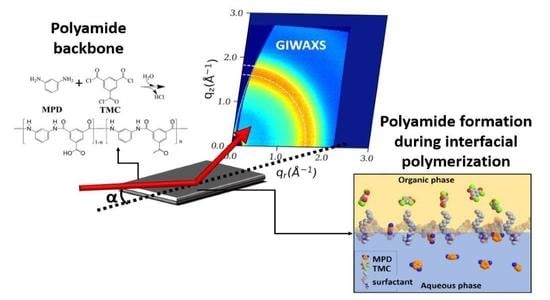
:1. Introduction
2. Ionic Liquid Selection
3. Experimental
3.1. Materials
3.2. IL-Mediated Interfacial Polymerization (IP) for RO Membrane Fabrication
3.3. RO Filtration Evaluation
3.4. Preparation of Free-Standing Thin Films
3.5. GIWAXS Characterization
3.6. Contact Angle Measurement
3.7. Zeta Potential Measurement
4. Results and Discussion
4.1. Performance Evaluation of IL-Mediated Interfacially Polymerized RO Membranes
4.2. GIWAXS Study of IL-Mediated IP PA Films
4.3. Water Contact Angle Measurements of IL-Mediated IP RO Membranes
4.4. Zeta Potentials of IL-Mediated IP RO Membranes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, R.J. Composite reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar]
- Korikov, A.P.; Kosaraju, P.B.; Sirkar, K.K. Interfacially polymerized hydrophilic microporous thin film composite membranes on porous polypropylene hollow fibers and flat films. J. Membr. Sci. 2006, 279, 588–600. [Google Scholar] [CrossRef]
- Racar, M.; Dolar, D.; Špehar, A.; Košutić, K. Application of UF/NF/RO membranes for treatment and reuse of rendering plant wastewater. Process Saf. Environ. Prot. 2017, 105, 386–392. [Google Scholar] [CrossRef]
- Alturki, A.A.; Tadkaew, N.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membr. Sci. 2010, 365, 206–215. [Google Scholar] [CrossRef]
- Nayar, K.G.; Lienhard, V.J.H. Brackish water desalination for greenhouse agriculture: Comparing the costs of RO, CCRO, EDR, and monovalent-selective EDR. Desalination 2020, 475, 114188. [Google Scholar] [CrossRef] [Green Version]
- Ramon, G.Z.; Hoek, E.M.V. Transport through composite membranes, part 2: Impacts of roughness on permeability and fouling. J. Membr. Sci. 2013, 425–426, 141–148. [Google Scholar] [CrossRef]
- Coronell, O.; Mi, B.; Mariñas, B.J.; Cahill, D.G. Modeling the effect of charge density in the active layers of reverse osmosis and nanofiltration membranes on the rejection of arsenic (III) and Potassium Iodide. Environ. Sci. Technol. 2013, 47, 420–428. [Google Scholar] [CrossRef]
- Gu, J.; Jun, B.; Kwon, Y. Effect of chlorination condition and permeability of chlorine species on the chlorination of a polyamide membrane. Water Res. 2012, 46, 5389–5400. [Google Scholar] [CrossRef]
- Tsai, H.A.; Li, L.D.; Lee, K.R.; Wang, Y.C.; Li, C.L.; Huang, J.; Lai, J.Y. Effect of surfactant addition on the morphology and pervaporation performance of asymmetric polysulfone membranes. J. Membr. Sci. 2000, 176, 97–103. [Google Scholar] [CrossRef]
- An, Q.; Li, F.; Ji, Y.; Chen, H. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes. J. Membr. Sci. 2011, 367, 158–165. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Zhang, X.; Zheng, G. Polyamide thin film composite membranes prepared from isomeric biphenyl tetraacyl chloride and m-phenylenediamine. J. Membr. Sci. 2008, 315, 20–27. [Google Scholar] [CrossRef]
- Tang, B.; Huo, X.; Wu, P. Study on a novel polyester composite nanofiltration membrane by interfacial polymerization of triethanolamine (TEOA) and trimesoyl chloride (TMC): I. Preparation, characterization and nanofiltration properties test of membrane. J. Membr. Sci. 2008, 320, 198–205. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Madaeni, S.S.; Rahimpour, A. Fabrication and development of interfacial polymerized thin-film composite nanofiltration membrane using different surfactants in organic phase; study of morphology and performance. J. Membr. Sci. 2009, 343, 219–228. [Google Scholar] [CrossRef]
- Mai, Z.; Butin, V.; Rakib, M.; Zhu, H.; Rabiller-Baudry, M.; Couallier, E. Influence of bulk concentration on the organisation of molecules at a membrane surface and flux decline during reverse osmosis of an anionic surfactant. J. Membr. Sci. 2016, 499, 257–268. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kimura, H. Fouling behaviour of a reverse osmosis membrane by three types of surfactants. J. Water Reuse Desalination 2012, 2, 40–46. [Google Scholar] [CrossRef]
- Baransi-Karkabyy, K.; Bass, M.; Freger, V. In situ modification of reverse osmosis membrane elements for enhanced removal of multiple micropollutants. Membranes 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baransi-Karkaby, K.; Bass, M.; Levchenko, S.; Eitan, S.; Freger, V. Facile modification of reverse osmosis membranes by surfactant-assisted acrylate grafting for enhanced selectivity. Environ. Sci. Technol. 2017, 51, 2347–2354. [Google Scholar] [CrossRef]
- Raval, H.D.; Raviya, M.R.; Rathod, H.C. Polyamide-surfactant interaction: Exploration of new avenues for reverse osmosis separation applications. Adv. Polym. Technol. 2018, 37, 3106–3114. [Google Scholar] [CrossRef]
- Chen, V.; Fane, A.G.; Fell, C.J.D. The use of anionic surfactants for reducing fouling of ultrafiltration membranes: Their effects and optimization. J. Membr. Sci. 1992, 67, 249–261. [Google Scholar] [CrossRef]
- Yamagiwa, K.; Kobayashi, H.; Onodera, M.; Ohkawa, A.; Kamiyama, Y.; Tasaka, K. Surfactant pretreatment of a polysulfone ultrafilter for reduction of antifoam fouling. Biotechnol. Bioeng. 1994, 43, 301. [Google Scholar] [CrossRef]
- Wilbert, M.C.; Pellegrino, J.; Zydney, A. Bench-scale testing of surfactant-modified reverse osmosis/nanofiltration membranes. Desalination 1998, 115, 15–32. [Google Scholar] [CrossRef]
- Roy, A.; Ghosh, A.; Datta, S.; Das, S.; Mohanraj, P.; Deb, J.; Bhanoji Rao, M.E. Effects of plasticizers and surfactants on the film forming properties of hydroxypropyl methylcellulose for the coating of diclofenac sodium tablets. Saudi Pharm. J. 2009, 17, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Hou, J.; Yan, Y.; Wang, J. The effect of surfactant adsorption on surface wettability and flow resistance in slit nanopore: A molecular dynamics study. J. Colloid Interface Sci. 2018, 513, 379–388. [Google Scholar] [CrossRef]
- Fu, Q.; Verma, N.; Hsiao, B.S.; Medellin-Rodriguez, F.; Beaucage, P.A.; Stafford, C.M.; Ocko, B.M. X-ray scattering studies of reverse osmosis materials. Synchrotron Radiat. News 2020, 33, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Verma, N.; Ma, H.; Medellin-Rodriguez, F.J.; Li, R.; Fukuto, M.; Stafford, C.M.; Hsiao, B.S.; Ocko, B.M. Molecular structure of aromatic reverse osmosis polyamide barrier Layers. ACS Macro Lett. 2019, 8, 352–356. [Google Scholar] [CrossRef]
- Foglia, F.; Karan, S.; Nania, M.; Jiang, Z.; Porter, A.E.; Barker, R.; Livingston, A.G.; Cabral, J.T. Neutron reflectivity and performance of polyamide nanofilms for water desalination. Adv. Funct. Mater. 2017, 27, 1701738. [Google Scholar] [CrossRef]
- Sunday, D.F.; Chan, E.P.; Orski, S.V.; Nieuwendaal, R.C.; Stafford, C.M. Functional group quantification of polymer nanomembranes with soft x-rays. Phys. Rev. Mater. 2018, 2, 032601. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.S.; Ray, P.; Xie, Z.; Hoang, M. Synchrotron SAXS to probe cross-linked network of polyamide ‘reverse osmosis’ and ‘nanofiltration’ membranes. J. Membr. Sci. 2012, 421–422, 51–59. [Google Scholar] [CrossRef]
- Pipich, V.; Schlenstedt, K.; Dickmann, M.; Kasher, R.; Meier-Haack, J.; Hugenschmidt, C.; Petry, W.; Oren, Y.; Schwahn, D. Morphology and porous structure of standalone aromatic polyamide films as used in RO membranes–An exploration with SANS, PALS, and SEM. J. Membr. Sci. 2019, 573, 167–176. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.-S. Solubilities of 1-hexyl-3-methylimidazole nitrate and 1-octyl-3-methylimidazole nitrate in selected solvents. J. Chem. Eng. Data 2011, 56, 4371–4375. [Google Scholar] [CrossRef]
- Pott, T.; Méléard, P. New insight into the nanostructure of ionic liquids: A small angle X-ray scattering (SAXS) study on liquid tri-alkyl-methyl-ammonium bis(trifluoromethanesulfonyl)amides and their mixtures. Phys. Chem. Chem. Phys. 2009, 11, 5469–5475. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.; Pontoni, D.; Murphy, B.M.; Festersen, S.; Runge, B.; Magnussen, O.M.; Steinruck, H.; Reichert, H.; Ocko, B.M.; Deutsch, M. Surface structure evolution in a homologous series of ionic liquids. Proc. Natl. Acad. Sci. USA 2018, 115, E1100–E1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, L.; Ma, H.; Wang, X.; Yoon, K.; Wang, R.; Hsiao, B.S.; Chu, B. Fabrication of thin-film nanofibrous composite membranes by interfacial polymerization using ionic liquids as additives. J. Membr. Sci. 2010, 365, 52–58. [Google Scholar] [CrossRef]
- Boyer, B.; Hambardzoumian, A.; Roque, J.; Beylerian, N. Inverse phase transfer catalysis versus interfacial catalysis. Effect of medium stirring in the epoxidation reaction of chalcone by hydrogen peroxide. J. Chem. Soc. Perkin Trans. 2 2002, 10, 1689–1691. [Google Scholar] [CrossRef]
- Jaramillo, H.; Boo, C.; Hashmi, S.M.; Elimelech, M. Zwitterionic coating on thin-film composite membranes to delay gypsum scaling in reverse osmosis. J. Membr. Sci. 2021, 618, 118568. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The Critical Need for Increased Selectivity, Not Increased Water Permeability, for Desalination Membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Annapureddy, H.V.R.; Kashyap, H.K.; de Biase, P.M.; Margulis, C.J. What is the origin of the prepeak in the X-ray scattering of imidazolium-based room-temperature ionic liquids. J. Phys. Chem. B 2010, 114, 16838–16846. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, L.; Zhao, H.; Ma, H.; Sajib, M.S.J.; Jiang, H.; Murad, S. Aromatic polyamide reverse-osmosis membrane: An atomistic molecular dynamics simulation. J. Phys. Chem. B 2016, 120, 10311–10318. [Google Scholar] [CrossRef]
- Liu, T.; Chen, D.; Yang, F.; Chen, J.; Cao, Y.; Xiang, M.; Kang, J.; Xu, R. Enhancing the permeability and anti-fouling properties of a polyamide thin-film composite reverse osmosis membrane via surface grafting of l-lysine. RSC Adv. 2019, 9, 20044–20052. [Google Scholar] [CrossRef]








| IL (in Thin PA Film) | Alkyl Chain Length | q1 (Å−1) | q2 (Å−1) | d1 (Å) | d2 (Å) | R (%) | F (L m2-h−1) |
|---|---|---|---|---|---|---|---|
| EMIC 1 | 2 | 1.62 | 1.83 | 3.8 | 3.6 | 98.32 | 22.89 |
| BMIC 2 | 4 | 1.61 | 1.82 | 3.9 | 3.4 | 95.29 | 27.07 |
| OMIC 3 | 8 | 1.61 | 1.81 | 3.9 | 3.4 | 92.34 | 34.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, N.; Chen, L.; Fu, Q.; Wu, S.; Hsiao, B.S. Ionic Liquid-Mediated Interfacial Polymerization for Fabrication of Reverse Osmosis Membranes. Membranes 2022, 12, 1081. https://doi.org/10.3390/membranes12111081
Verma N, Chen L, Fu Q, Wu S, Hsiao BS. Ionic Liquid-Mediated Interfacial Polymerization for Fabrication of Reverse Osmosis Membranes. Membranes. 2022; 12(11):1081. https://doi.org/10.3390/membranes12111081
Chicago/Turabian StyleVerma, Nisha, Lexin Chen, Qinyi Fu, Skyler Wu, and Benjamin S. Hsiao. 2022. "Ionic Liquid-Mediated Interfacial Polymerization for Fabrication of Reverse Osmosis Membranes" Membranes 12, no. 11: 1081. https://doi.org/10.3390/membranes12111081
APA StyleVerma, N., Chen, L., Fu, Q., Wu, S., & Hsiao, B. S. (2022). Ionic Liquid-Mediated Interfacial Polymerization for Fabrication of Reverse Osmosis Membranes. Membranes, 12(11), 1081. https://doi.org/10.3390/membranes12111081