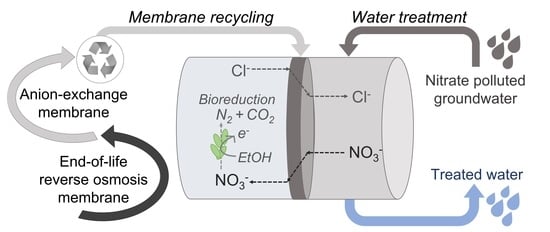
Nitrate Removal by Donnan Dialysis and Anion-Exchange Membrane Bioreactor Using Upcycled End-of-Life Reverse Osmosis Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Membranes
2.3. Confocal Micro-Raman Spectroscopy
2.4. Donnan Dialysis (DD) and Ion-Exchange Membrane Bioreactor (IEMB) Experiments
2.4.1. Model Solutions, Test Cell, and Analytical Methods
2.4.2. Operation as a Donnan Dialyzer
2.4.3. Operation as an Ion-Exchange Membrane Bioreactor
Microbial Culture
Ethanol Permeation Studies
Operation as a Bioreactor
2.4.4. Analytical Methods
2.5. 2D Fluorescence Spectroscopy
- Rinse with DI water
- Passive immersion in 5000 mg L−1 NaOH aqueous solution for 10 min, at room temperature.
- Rinse with DI water
- Passive immersion in a 20,000 mg L−1 citric acid aqueous solution for 30 min, at room temperature.
- Rinse with DI water
3. Results
3.1. Confocal Micro-Raman Spectroscopy
3.2. Donnan Dialysis (DD) and Ion-Exchange Membrane Bioreactor (IEMB) Experiments
3.2.1. Time Course Concentration of Nitrate in the Feed and in the Receiver Compartments
3.2.2. Co-Ion Leakage to the Feed Compartment
3.2.3. Diffusion Permeability of the Carbon Source
3.3. 2D Fluorescence Spectroscopy
3.4. Preliminary Membrane-Associated Costs and Environmental Implications
4. Conclusions
- µ Raman spectroscopy confirmed the successful deposition of the ion-exchange resin in the coated (top) surface of the membrane. However, the analysis revealed an asymmetric distribution of the ion-exchange sites in the membrane cross-section, which might contribute to the relatively low ion-exchange capacity and water content and the relatively high electrical resistance of the membranes and, therefore, have a great influence on transport properties of the membrane.
- In respect to nitrate removal under DD and IEMB operation, the membrane incorporating Purolite® A600/9149 ion-exchange resin (Pur-RE membrane) achieved, among the prepared membranes, the best removal yields (57% and 56% of nitrate removal within 24 h under DD and IEMB operation, respectively), probably due to its higher ion-exchange capacity and lower electrical resistance, in comparison with the Amb-RE and the Lew-RE membranes. Furthermore, nitrate was biologically eliminated in the IEMB, favouring the reuse of the receiver solution and avoiding the discharge of the pollutant into the environment.
- 2D fluorescence spectroscopy can effectively detect alterations in the excitation emission spectra of the membranes, caused by fouling and/or chemical cleaning. In this respect, the Pur-RE membrane was found to be the most stable membrane under the tested conditions.
- The relative low cost of the employed materials in the formulation of Pur-Re membrane anticipated an economic benefit of the presented membrane recycling concept.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mayor, B. Growth patterns in mature desalination technologies and analogies with the energy field. Desalination 2019, 457, 75–84. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Landaburu-Aguirre, J.; García-Pacheco, R.; Molina, S.; Rodríguez-Sáez, L.; Rabadán, J.; García-Calvo, E. Fouling prevention, preparing for re-use and membrane recycling. Towards circular economy in RO desalination. Desalination 2016, 393, 16–30. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular economy in membrane technology: Using end-of-life reverse osmosis modules for preparation of recycled anion exchange membranes and validation in electrodialysis. J. Memb. Sci. 2020, 593, 117423. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Riccardelli, G.; García-Calvo, E. Influence of acid/base activation treatment in the performance of recycled electromembrane for fresh water production by electrodialysis. Chemosphere 2020, 248, 126027. [Google Scholar] [CrossRef] [PubMed]
- Lejarazu-Larrañaga, A.; Ortiz, J.M.; Molina, S.; Zhao, Y.; García-Calvo, E. Nitrate-selective anion exchange membranes prepared using discarded reverse osmosis membranes as support. Membranes 2020, 10, 377. [Google Scholar] [CrossRef]
- Tanaka, Y. Ion Exchange Membranes, Fundamentals and Applications, 1st ed.; Tanaka, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-51982-5. [Google Scholar]
- Zhao, B.; Zhao, H.; Dockko, S.; Ni, J. Arsenate removal from simulated groundwater with a Donnan dialyzer. J. Hazard. Mater. 2012, 215–216, 159–165. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Kweinor Tetteh, E.; Ezugbe, E.O.; Pillay, L.V. Donnan membrane process for the selective recovery and removal of target metal ions—A mini review. Membranes 2021, 11, 358. [Google Scholar] [CrossRef]
- Crespo, J.G.; Velizarov, S.; Reis, M.A. Membrane bioreactors for the removal of anionic micropollutants from drinking water. Curr. Opin. Biotechnol. 2004, 15, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bijay-Singh; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Vo, T.K.Q.; Lee, J.J.; Kang, J.S.; Park, S.; Kim, H.S. Nitrogen removal by sulfur-based carriers in a membrane bioreactor (MBR). Membranes 2018, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241549950. [Google Scholar]
- Schoeman, J.J.; Steyn, A. Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 2003, 155, 15–26. [Google Scholar] [CrossRef]
- Elmidaoui, A.; Elhannouni, F.; Sahli, M.A.M.; Chay, L.; Elabbassi, H. Pollution of nitrate in Moroccan ground water: Removal by electrodialysis. Desalination 2001, 136, 325–332. [Google Scholar] [CrossRef]
- Samatya, S.; Kabay, N.; Yüksel, Ü.; Arda, M.; Yüksel, M. Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React. Funct. Polym. 2006, 66, 1206–1214. [Google Scholar] [CrossRef]
- Aslan, S.; Cakici, H. Biological denitrification of drinking water in a slow sand filter. J. Hazard. Mater. 2007, 148, 253–258. [Google Scholar] [CrossRef]
- Huno, S.K.M.; Rene, E.R.; Van Hullebusch, E.D.; Annachhatre, A.P. Nitrate removal from groundwater: A review of natural and engineered processes. J. Water Supply Res. Technol. Aqua 2018, 67, 885–902. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.I.M. Biological denitrification of groundwater. Water Air Soil Pollut. 2000, 123, 183–193. [Google Scholar] [CrossRef]
- Velizarov, S.; Crespo, J.G.; Reis, M.A. Ion exchange membrane bioreactor for selective removal of nitrate from drinking water: Control of ion fluxes and process performance. Biotechnol. Prog. 2002, 18, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.D.; Crespo, J.G.; Almeida, J.S.; Reis, M.A. Drinking water denitrification using a novel ion-exchange membrane bioreactor. Environ. Sci. Technol. 2000, 34, 1557–1562. [Google Scholar] [CrossRef]
- Matos, C.T.; Fortunato, R.; Velizarov, S.; Reis, M.A.M.; Crespo, G.Ã. Removal of mono-valent oxyanions from water in an ion exchange membrane bioreactor: Influence of membrane permselectivity. Water Res. 2008, 42, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sáez, L.; Landaburu-Aguirre, J.; Molina, S.; García-Payo, M.C.; García-Calvo, E. Study of surface modification of recycled ultrafiltration membranes using statistical design of experiments. Surf. Interfaces 2021, 23, 100978. [Google Scholar] [CrossRef]
- Campos Pozuelo, E.; Terrero Rodríguez, P.; Zarzo Martínez, D.; Molina Serrano, F.J.; Calzada Garzón, M.; García Pacheco, R.; Molina Martínez, S.; Rodríguez Sáez, L.; Rabadán, F.J.; Landaburu Aguirre, J.; et al. Transformation of Spiral Wound Polyamide Membranes after Its Industrial Lifespan. Spanish Patent PCT/EP2016/30931, 8 July 2016. [Google Scholar]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Terrero-Rodríguez, P.; Campos, E.; Molina-Serrano, F.; Rabadán, J.; Zarzo, D.; García-Calvo, E. Validation of recycled membranes for treating brackish water at pilot scale. Desalination 2018, 433, 199–208. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Molina, S.; Rodríguez-Sáez, L.; Teli, S.B.; García-Calvo, E. Transformation of end-of-life RO membranes into NF and UF membranes: Evaluation of membrane performance. J. Memb. Sci. 2015, 495, 305–315. [Google Scholar] [CrossRef]
- Molina, S.; Landaburu-Aguirre, J.; Rodríguez-Sáez, L.; García-Pacheco, R.; de la Campa, J.G.; García-Calvo, E. Effect of sodium hypochlorite exposure on polysulfone recycled UF membranes and their surface characterization. Polym. Degrad. Stab. 2018, 150, 46–56. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Lejarazu-Larrañaga, A.; Rodríguez-Sáez, L.; Molina, S.; Ransome, T.; García-Calvo, E. Free chlorine exposure dose (ppm·h) and its impact on RO membranes ageing and recycling potential. Desalination 2019, 457, 133–143. [Google Scholar] [CrossRef]
- Mega Group Companies Product Data Sheet. Ralex® Membrane AMHPES. Available online: https://www.mega.cz/files/datasheet/MEGA-RALEX-AMH-PES-en.pdf (accessed on 3 June 2021).
- Ricardo, A.R.; Velizarov, S.; Crespo, J.G.; Reis, M.A.M. Validation of the ion-exchange membrane bioreactor concept in a plate-and-frame module configuration. Process. Biochem. 2012, 47, 1832–1838. [Google Scholar] [CrossRef]
- Matos, C.T.; Velizarov, S.; Crespo, J.G.; Reis, M.A.M. Simultaneous removal of perchlorate and nitrate from drinking water using the ion exchange membrane bioreactor concept. Water Res. 2006, 40, 231–240. [Google Scholar] [CrossRef]
- Pawlowski, S.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. 2D fluorescence spectroscopy for monitoring ion-exchange membrane based technologies—Reverse electrodialysis (RED). Water Res. 2016, 88, 184–198. [Google Scholar] [CrossRef]
- Velizarov, S. Transport of arsenate through anion-exchange membranes in Donnan dialysis. J. Memb. Sci. 2013, 425–426, 243–250. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. APHA Method 4500-NO3: Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington DC, USA, 1992; Volume 552, ISBN 0-87553-207-1. [Google Scholar]
- Pawlowski, S.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. Prediction of reverse electrodialysis performance by inclusion of 2D fluorescence spectroscopy data into multivariate statistical models. Sep. Purif. Technol. 2015, 150, 159–169. [Google Scholar] [CrossRef]
- Golubenko, D.; Yaroslavtsev, A. Development of surface-sulfonated graft anion-exchange membranes with monovalent ion selectivity and antifouling properties for electromembrane processes. J. Memb. Sci. 2020, 612, 118408. [Google Scholar] [CrossRef]
- Bro, R. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Hummel, D.O. Atlas of Plastics Additives. Analysis by Spectrometric Methods; Springer: Berlin, Germany, 2002. [Google Scholar]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef]
- Pyrzynska, K. Preconcentration and recovery of metal ions by Donnan dialysis. Microchim. Acta 2006, 153, 117–126. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic mobility in ion-exchange membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef]
- Velizarov, S.; Rodrigues, C.M.; Reis, M.A.; Crespo, J.G. Mechanism of charged pollutants removal in an ion exchange membrane bioreactor: Drinking water denitrification. Biotechnol. Bioeng. 2000, 71, 245–254. [Google Scholar] [CrossRef]
- Yurova, P.A.; Karavanova, Y.A.; Gorbunova, Y.G.; Yaroslavtsev, A.B. Transport properties of asymmetric ion-exchange membranes based on MC-40, MF-4SC, and polyaniline. Pet. Chem. 2014, 54, 551–555. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Nemati, M.; Jeddi, F.; Salehi, E.; Khodabakhshi, A.R.; Madaeni, S.S. Fabrication of mixed matrix heterogeneous cation exchange membrane modified by titanium dioxide nanoparticles: Mono/bivalent ionic transport property in desalination. Desalination 2015, 359, 167–175. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Jeddi, F.; Nemati, M.; Madaeni, S.S.; Moghadassi, A.R. Electrodialysis heterogeneous anion exchange membrane modified by PANI/MWCNT composite nanoparticles: Preparation, characterization and ionic transport property in desalination. Desalination 2014, 341, 107–114. [Google Scholar] [CrossRef]
- Mubita, T.; Porada, S.; Aerts, P.; Wal, A. Van Der Heterogeneous anion exchange membranes with nitrate selectivity and low electrical resistance. J. Memb. Sci. 2020, 607, 118000. [Google Scholar] [CrossRef]
- Breytus, A.; Hasson, D.; Semiat, R.; Shemer, H. Ion exchange membrane adsorption in Donnan dialysis. Sep. Purif. Technol. 2019, 226, 252–258. [Google Scholar] [CrossRef]
- Galinha, C.F.; Carvalho, G.; Portugal, C.A.M.; Guglielmi, G.; Reis, M.A.M.; Crespo, J.G. Two-dimensional fluorescence as a fingerprinting tool for monitoring wastewater treatment systems. J. Chem. Technol. Biotechnol. 2011, 86, 985–992. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; García-Pacheco, R.; Landaburu-Aguirre, J.; García-Calvo, E. Recycling of end-of-life reverse osmosis membranes: Comparative LCA and cost-effectiveness analysis at pilot scale. Resour. Conserv. Recycl. 2019, 150, 104423. [Google Scholar] [CrossRef]
- Melnikov, S.; Loza, S.; Sharafan, M.; Zabolotskiy, V. Electrodialysis treatment of secondary steam condensate obtained during production of ammonium nitrate. Technical and economic analysis. Sep. Purif. Technol. 2016, 157, 179–191. [Google Scholar] [CrossRef]
- Fetting, C. The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. Circular Economy Action Plan. For a cleaner and more competitive Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- Galinha, C.F.; Carvalho, G.; Portugal, C.A.M.; Guglielmi, G.; Oliveira, R.; Crespo, J.G.; Reis, M.A.M. Real-time monitoring of membrane bioreactors with 2D-fluorescence data and statistically based models. Water Sci. Technol. 2011, 63, 1381–1388. [Google Scholar] [CrossRef]
- Zaman, M.; Liu, H.; Xiao, H.; Chibante, F.; Ni, Y. Hydrophilic modification of polyester fabric by applying nanocrystalline cellulose containing surface finish. Carbohydr. Polym. 2013, 91, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Tetrahydrofuran Market Price & Analysis. Available online: https://www.echemi.com/productsInformation/pd20150901266-tetrahydrofuran.html (accessed on 7 September 2021).
- Statista Price of Polyvinyl Chloride Worldwide from 2017 to 2020 with Estimated Figures for 2021 to 2022. Available online: https://www.statista.com/statistics/1171131/price-polyvinyl-chloride-forecast-globally/#statisticContainer (accessed on 6 September 2021).







| Anion-Exchange Membrane | AMH-PES | Pur-RE | Amb-RE | Lew-RE |
|---|---|---|---|---|
| Mechanical support | Polyester (PET) | End-of-life RO | End-of-life RO | End-of-life RO |
| Polymer binder | Polyethylene (PE) | PVC | PVC | PVC |
| Ion-exchange resin | Unspecified | Purolite® A600/9149 | Amberlite® IRA-402 | Lewatit® Sybron Ionac® SR-7 |
| Ion-exchange group | R—(CH3)3N+ | R—(CH3)3N+ | R—(CH3)3N+ | R—(C3H7)3N+ |
| Membrane thickness (µm) | 645 ± 5 | 190 ± 4 | 184 ± 7 | 182 ± 7 |
| Water content (%) a | 50 ± 0 | 23 ± 4 | 21 ± 4 | 19 ± 1 |
| IEC (mmol g−1) b | 2.19 ± 0.09 | 0.75 ± 0.14 | 0.62 ± 0.04 | 0.39 ± 0.01 |
| Permselectivity (%) c | 81 ± 1 | 65 ± 9 | 83 ± 7 | 66 ± 4 |
| Electrical resistance (Ω cm2) d | 19 ± 3 | 56 ± 7 | 129 ± 1 | 120 ± 11 |
| Pur-RE | AMH-PES | Pur-RE Membrane Surface (m2) Equivalent to Performance of 1 m2 AMH-PES * | Pur-RE Membrane Cost (EUR) Equivalent to Performance of 1 m2 AMH-PES ** | |
|---|---|---|---|---|
| (g m−2 h−1) in DD | 0.82 | 6.34 | 7.73 | 25.2 |
| (g m−2 h−1) in DD | 0.18 | 2.70 | 14.81 | 48.3 |
| (g m−2 h−1) in the IEMB | 0.81 | 6.00 | 7.39 | 24.1 |
| (g m−2 h−1) in the IEMB | 0.19 | 2.61 | 13.50 | 44.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejarazu-Larrañaga, A.; Ortiz, J.M.; Molina, S.; Pawlowski, S.; Galinha, C.F.; Otero, V.; García-Calvo, E.; Velizarov, S.; Crespo, J.G. Nitrate Removal by Donnan Dialysis and Anion-Exchange Membrane Bioreactor Using Upcycled End-of-Life Reverse Osmosis Membranes. Membranes 2022, 12, 101. https://doi.org/10.3390/membranes12020101
Lejarazu-Larrañaga A, Ortiz JM, Molina S, Pawlowski S, Galinha CF, Otero V, García-Calvo E, Velizarov S, Crespo JG. Nitrate Removal by Donnan Dialysis and Anion-Exchange Membrane Bioreactor Using Upcycled End-of-Life Reverse Osmosis Membranes. Membranes. 2022; 12(2):101. https://doi.org/10.3390/membranes12020101
Chicago/Turabian StyleLejarazu-Larrañaga, Amaia, Juan M. Ortiz, Serena Molina, Sylwin Pawlowski, Claudia F. Galinha, Vanessa Otero, Eloy García-Calvo, Svetlozar Velizarov, and João G. Crespo. 2022. "Nitrate Removal by Donnan Dialysis and Anion-Exchange Membrane Bioreactor Using Upcycled End-of-Life Reverse Osmosis Membranes" Membranes 12, no. 2: 101. https://doi.org/10.3390/membranes12020101
APA StyleLejarazu-Larrañaga, A., Ortiz, J. M., Molina, S., Pawlowski, S., Galinha, C. F., Otero, V., García-Calvo, E., Velizarov, S., & Crespo, J. G. (2022). Nitrate Removal by Donnan Dialysis and Anion-Exchange Membrane Bioreactor Using Upcycled End-of-Life Reverse Osmosis Membranes. Membranes, 12(2), 101. https://doi.org/10.3390/membranes12020101