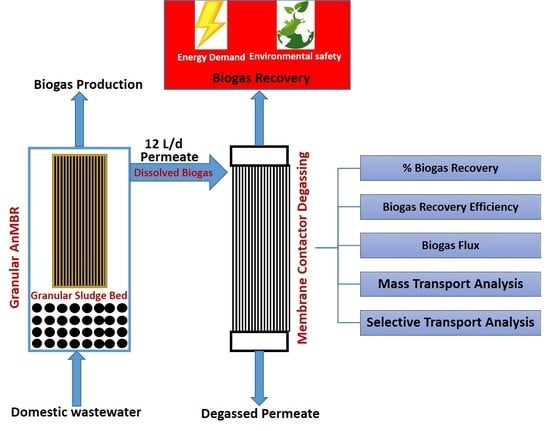
Hollow-Fiber Membrane Contactor for Biogas Recovery from Real Anaerobic Membrane Bioreactor Permeate
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Effluent Preparation and Analysis
2.3. Degassing
2.4. Determination of the Desorbed and Dissolved Biogas
2.5. Performance Evaluation and Mass Transfer Calculations
3. Results and Discussion
3.1. Degassing System Performance
3.2. Membrane Degassing System Efficiency
3.3. Analysis of Biogas Flux
3.4. Experimental and Theoretical Mass Transfer Analysis: Pseudo-Wetting Prediction
3.5. Real AnMBR Permeate Dissolved Biogas Degassing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Parameters for Local Mass Transfer Calculations
| Parameter | Specie | Equation | Ref. |
|---|---|---|---|
| Gas diffusivity (m2 s−1) (in gas phase) | CO2,CH4,N2 | (1 ,2 = Gas components) | [59] |
| Mix-effective | , (1, 2, 3 … n = gas species) | ||
| Gas diffusivity (m2 s−1) (in liquid phase) | CO2, CH4 | (1 = Gas solute, 2 = Liquid solvent) | |
| Gas diffusivity (m2 s−1) (porous membrane) | CO2, CH4 | [45] | |
| Density (Kg m−3) | Gas mixture | Ideal Gas Law | |
| Water | [60] | ||
| Viscosity (Kg m−1 s−1) | Gas mixture | [61] | |
| Water | [60] |
References
- McCarty, P.L.; Bae, J.; Kim, J. Domestic Wastewater Treatment as a Net Energy Producer-Can This Be Achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic Membrane Bioreactors for Wastewater Treatment: Novel Configurations, Fouling Control and Energy Considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Zhang, J.; Liang, S.; Ni, B.J.; Zhang, X.B.; Wang, J. Comparing the Value of Bioproducts from Different Stages of Anaerobic Membrane Bioreactors. Bioresour. Technol. 2016, 214, 816–825. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane Bioreactors—A Review on Recent Developments in Energy Reduction, Fouling Control, Novel Configurations, LCA and Market Prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef] [Green Version]
- Lettinga, G.; Rebac, S.; Parshina, S.; Nozhevnikova, A.; Van Lier, J.B.; Stams, A.J.M. High-Rate Anaerobic Treatment of Wastewater at Low Temperatures. Appl. Environ. Microbiol. 1999, 65, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Angenent, L.T.; Banik, G.C.; Sung, S. Anaerobic Migrating Blanket Reactor Treatment of Low-Strength Wastewater at Low Temperatures. Water Environ. Res. 2001, 73, 567–574. [Google Scholar] [CrossRef]
- Bandara, W.M.K.R.T.W.; Satoh, H.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Removal of Residual Dissolved Methane Gas in an Upflow Anaerobic Sludge Blanket Reactor Treating Low-Strength Wastewater at Low Temperature with Degassing Membrane. Water Res. 2011, 45, 3533–3540. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, Q.; Zheng, S.; Wu, D. Anaerobic Membrane Bioreactor (AnMBR) for Bamboo Industry Wastewater Treatment. Bioresour. Technol. 2013, 149, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Gao, X.; Wang, C.; Xu, X.; Zhu, L. An Enhanced Anaerobic Membrane Bioreactor Treating Bamboo Industry Wastewater by Bamboo Charcoal Addition: Performance and Microbial Community Analysis. Bioresour. Technol. 2016, 220, 26–33. [Google Scholar] [CrossRef]
- Aslam, M.; McCarty, P.L.; Shin, C.; Bae, J.; Kim, J. Low Energy Single-Staged Anaerobic Fluidized Bed Ceramic Membrane Bioreactor (AFCMBR) for Wastewater Treatment. Bioresour. Technol. 2017, 240, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ahmad, R.; Yasin, M.; Khan, A.L.; Shahid, M.K.; Hossain, S.; Khan, Z.; Jamil, F.; Rafiq, S.; Bilad, M.R.; et al. Anaerobic Membrane Bioreactors for Biohydrogen Production: Recent Developments, Challenges and Perspectives. Bioresour. Technol. 2018, 269, 452–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, B.Q.; Kraemer, J.T.; Bagley, D.M. Anaerobic Membrane Bioreactors: Applications and Research Directions. Crit. Rev. Environ. Sci. Technol. 2006, 36, 489–530. [Google Scholar] [CrossRef]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous Hydrogen Utilization and in Situ Biogas Upgrading in an Anaerobic Reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef]
- Luo, G.; Wang, W.; Angelidaki, I. A New Degassing Membrane Coupled Upflow Anaerobic Sludge Blanket (UASB) Reactor to Achieve in-Situ Biogas Upgrading and Recovery of Dissolved CH4 from the Anaerobic Effluent. Appl. Energy 2014, 132, 536–542. [Google Scholar] [CrossRef]
- Daelman, M.R.J.; van Voorthuizen, E.M.; van Dongen, U.G.J.M.; Volcke, E.I.P.; van Loosdrecht, M.C.M. Methane Emission during Municipal Wastewater Treatment. Water Res. 2012, 46, 3657–3670. [Google Scholar] [CrossRef] [PubMed]
- Cookney, J.; Cartmell, E.; Jefferson, B.; McAdam, E.J. Recovery of Methane from Anaerobic Process Effluent Using Poly-Di-Methyl-Siloxane Membrane Contactors. Water Sci. Technol. 2012, 65, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Crone, B.C.; Garland, J.L.; Sorial, G.A.; Vane, L.M. Significance of Dissolved Methane in Effluents of Anaerobically Treated Low Strength Wastewater and Potential for Recovery as an Energy Product: A Review. Water Res. 2016, 104, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Blasing, T.J. Recent Greenhouse Gas Concentrations; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2009. [Google Scholar]
- Heile, S.; Chernicharo, C.A.L.; Brandt, E.M.F.; McAdam, E.J. Dissolved Gas Separation for Engineered Anaerobic Wastewater Systems. Sep. Purif. Technol. 2017, 189, 405–418. [Google Scholar] [CrossRef] [Green Version]
- Stanojević, M.; Lazarević, B.; Radić, D. Review of Membrane Contactors Designs and Applications of Different Modules in Industry. FME Trans. 2003, 31, 91–98. [Google Scholar]
- Chen, S.; Smith, A.L. Methane-Driven Microbial Fuel Cells Recover Energy and Mitigate Dissolved Methane Emissions from Anaerobic Effluents. Environ. Sci. Water Res. Technol. 2018, 4, 67–79. [Google Scholar] [CrossRef]
- Sethunga, G.S.M.D.P.; Rongwong, W.; Wang, R.; Bae, T.H. Optimization of Hydrophobic Modification Parameters of Microporous Polyvinylidene Fluoride Hollow-Fiber Membrane for Biogas Recovery from Anaerobic Membrane Bioreactor Effluent. J. Membr. Sci. 2018, 548, 510–518. [Google Scholar] [CrossRef]
- Younas, M.; Tahir, T.; Wu, C.; Farrukh, S.; Sohaib, Q.; Muhammad, A.; Rezakazemi, M.; Li, J. Post-Combustion CO2 capture with Sweep Gas in Thin Film Composite (TFC) Hollow Fiber Membrane (HFM) Contactor. J. CO2 Util. 2020, 40, 101266. [Google Scholar] [CrossRef]
- Henares, M.; Izquierdo, M.; Marzal, P.; Martínez-Soria, V. Demethanization of Aqueous Anaerobic Effluents Using a Polydimethylsiloxane Membrane Module: Mass Transfer, Fouling and Energy Analysis. Sep. Purif. Technol. 2017, 186, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Rongwong, W.; Goh, K.; Sethunga, G.S.M.D.P.; Bae, T.H. Fouling Formation in Membrane Contactors for Methane Recovery from Anaerobic Effluents. J. Membr. Sci. 2019, 573, 534–543. [Google Scholar] [CrossRef]
- Sohaib, Q.; Muhammad, A.; Younas, M.; Rezakazemi, M.; Druon-Bocquet, S.; Sanchez-Marcano, J. Rigorous Non-Isothermal Modeling Approach for Mass and Energy Transport during CO2 Absorption into Aqueous Solution of Amino Acid Ionic Liquids in Hollow Fiber Membrane Contactors. Sep. Purif. Technol. 2021, 254, 117644. [Google Scholar] [CrossRef]
- Yan, S.P.; Fang, M.X.; Zhang, W.F.; Wang, S.Y.; Xu, Z.K.; Luo, Z.Y.; Cen, K.F. Experimental Study on the Separation of CO2 from Flue Gas Using Hollow Fiber Membrane Contactors without Wetting. Fuel Process. Technol. 2007, 88, 501–511. [Google Scholar] [CrossRef]
- Sohaib, Q.; Vadillo, J.M.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. Post-Combustion CO2 Capture by Coupling [Emim] Cation Based Ionic Liquids with a Membrane Contactor; Pseudo-Steady-State Approach. Int. J. Greenh. Gas Control 2020, 99, 103076. [Google Scholar] [CrossRef]
- Qazi, S.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Younas, M.; Sanchez-Marcano, J. Mathematical Modeling of CO 2 Absorption with Ionic Liquids in a Membrane Contactor, Study of Absorption Kinetics and Influence of Temperature. J. Chem. Technol. Biotechnol. 2019, 95, 1844–1857. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Novel Method for Incorporating Hydrophobic Silica Nanoparticles on Polyetherimide Hollow Fiber Membranes for CO2 Absorption in a Gas-Liquid Membrane Contactor. J. Membr. Sci. 2014, 452, 379–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Gas-Liquid Membrane Contactors for Acid Gas Removal: Recent Advances and Future Challenges. Curr. Opin. Chem. Eng. 2013, 2, 255–262. [Google Scholar] [CrossRef]
- Sethunga, G.S.M.D.P.; Karahan, H.E.; Wang, R.; Bae, T.H. PDMS-Coated Porous PVDF Hollow Fiber Membranes for Efficient Recovery of Dissolved Biomethane from Anaerobic Effluents. J. Membr. Sci. 2019, 584, 333–342. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, B.; Zhou, X.; He, Y.; Hong, H.; Lin, H.; Chen, J. Effects of Hydrophilicity/Hydrophobicity of Membrane on Membrane Fouling in a Submerged Membrane Bioreactor. Bioresour. Technol. 2015, 175, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Mulcahy, D.; Zou, L.; Kim, I.S.; Le-Clech, P. Influence of Hydrophobic and Electrostatic Membrane Surface Properties on Biofouling in a Submerged Membrane Bioreactor under Different Filtration Modes. Desalination Water Treat. 2016, 57, 26641–26647. [Google Scholar] [CrossRef]
- Hashemifard, S.A.; Ismail, A.F.; Matsuura, T.; Dashtarzhandi, M.R. Performance of Silicon Rubber Coated Polyetherimide Hollow Fibers for CO2 Removal via a Membrane Contactor. RSC Adv. 2015, 5, 48442–48455. [Google Scholar] [CrossRef]
- Sanchis-Perucho, P.; Robles, Á.; Durán, F.; Ferrer, J.; Seco, A. PDMS Membranes for Feasible Recovery of Dissolved Methane from AnMBR Effluents. J. Membr. Sci. 2020, 604, 118070. [Google Scholar] [CrossRef]
- Jiménez-Robles, R.; Gabaldón, C.; Martínez-Soria, V.; Izquierdo, M. Simultaneous Application of Vacuum and Sweep Gas in a Polypropylene Membrane Contactor for the Recovery of Dissolved Methane from Water. J. Membr. Sci. 2020, 617, 118560. [Google Scholar] [CrossRef]
- Jiménez-Robles, R.; Gabaldón, C.; Badia, J.D.; Izquierdo, M.; Martínez-Soria, V. Recovery of Dissolved Methane through a Flat Sheet Module with PDMS, PP, and PVDF Membranes. Sep. Purif. Technol. 2022, 282, 120057. [Google Scholar] [CrossRef]
- Layer, M.; Adler, A.; Reynaert, E.; Hernandez, A.; Pagni, M.; Morgenroth, E.; Holliger, C.; Derlon, N. Organic Substrate Diffusibility Governs Microbial Community Composition, Nutrient Removal Performance and Kinetics of Granulation of Aerobic Granular Sludge. Water Res. X 2019, 4, 100033. [Google Scholar] [CrossRef]
- Lahdhiri, A.; Sid, S.; Lesage, G.; Heran, M. Impact of Decreasing COD/N Ratio on Nitrogen Removal and Fouling in a Membrane Bioreactor for Urban Wastewater Treatment. Desalination Water Treat. 2017, 80, 121–132. [Google Scholar] [CrossRef]
- Iversen, S.B.; Bhatia, V.K.; Dam-Johansen, K.; Jonsson, G. Characterization of Microporous Membranes for Use in Membrane Contactors. J. Membr. Sci. 1997, 130, 205–217. [Google Scholar] [CrossRef]
- Yu, Y.; Ramsay, J.A.; Ramsay, B.A. On-Line Estimation of Dissolved Methane Concentration during Methanotrophic Fermentations. Biotechnol. Bioeng. 2006, 95, 788–793. [Google Scholar] [CrossRef]
- Henares, M.; Ferrero, P.; San-Valero, P.; Martínez-Soria, V.; Izquierdo, M. Performance of a Polypropylene Membrane Contactor for the Recovery of Dissolved Methane from Anaerobic Effluents: Mass Transfer Evaluation, Long-Term Operation and Cleaning Strategies. J. Membr. Sci. 2018, 563, 926–937. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Qazi, S.; Manuel Vadillo, J.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 Capture with Room Temperature Ionic Liquids; Coupled Absorption/Desorption and Single Module Absorption in Membrane Contactor. Chem. Eng. Sci. 2020, 223, 115719. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.M.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and Progress of Membrane Contactors in Post-Combustion Carbon Capture: A State-of-the-Art Review of New Developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- Shen, S.; Kentish, S.E.; Stevens, G.W. Shell-Side Mass-Transfer Performance in Hollow-Fiber Membrane Contactors. Solvent Extr. Ion Exch. 2010, 28, 817–844. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S.-T. Hollow Fiber Membrane Contactors. J. Membr. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Cookney, J.; Mcleod, A.; Mathioudakis, V.; Ncube, P.; Soares, A.; Jefferson, B.; McAdam, E.J. Dissolved Methane Recovery from Anaerobic Effluents Using Hollow Fibre Membrane Contactors. J. Membr. Sci. 2016, 502, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Wongchitphimon, S.; Rongwong, W.; Chuah, C.Y.; Wang, R.; Bae, T.H. Polymer-Fluorinated Silica Composite Hollow Fiber Membranes for the Recovery of Biogas Dissolved in Anaerobic Effluent. J. Membr. Sci. 2017, 540, 146–154. [Google Scholar] [CrossRef]
- McLeod, A.; Jefferson, B.; McAdam, E.J. Toward Gas-Phase Controlled Mass Transfer in Micro-Porous Membrane Contactors for Recovery and Concentration of Dissolved Methane in the Gas Phase. J. Membr. Sci. 2016, 510, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Mosadegh-Sedghi, S.; Rodrigue, D.; Brisson, J.; Iliuta, M.C. Wetting Phenomenon in Membrane Contactors—Causes and Prevention. J. Membr. Sci. 2014, 452, 332–353. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.Y.; Feron, P.H.M.; Liang, D.T. Influence of Membrane Wetting on CO2 Capture in Microporous Hollow Fiber Membrane Contactors. Sep. Purif. Technol. 2005, 46, 33–40. [Google Scholar] [CrossRef]
- Qazi, S.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 Capture in a Hollow Fiber Membrane Contactor Coupled with Ionic Liquid: Influence of Membrane Wetting and Process Parameters. Sep. Purif. Technol. 2020, 233, 115986. [Google Scholar] [CrossRef]
- Galib, M.; Elbeshbishy, E.; Reid, R.; Hussain, A.; Lee, H.S. Energy-Positive Food Wastewater Treatment Using an Anaerobic Membrane Bioreactor (AnMBR). Environ. Manag. 2016, 182, 477–485. [Google Scholar] [CrossRef]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Psychrophilic Anaerobic Membrane Bioreactor Treatment of Domestic Wastewater. Water Res. 2013, 47, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Ng, K.K.; Hong, P.K.A.; Yang, P.Y.; Lin, C.F. Treatment of Low-Strength Wastewater at Mesophilic and Psychrophilic Conditions Using Immobilized Anaerobic Biomass. Chem. Eng. J. 2017, 311, 46–54. [Google Scholar] [CrossRef]
- Yeo, H.; An, J.; Reid, R.; Rittmann, B.E.; Lee, H.S. Contribution of Liquid/Gas Mass-Transfer Limitations to Dissolved Methane Oversaturation in Anaerobic Treatment of Dilute Wastewater. Environ. Sci. Technol. 2015, 49, 10366–10372. [Google Scholar] [CrossRef]
- James, R.W.; Gregory, L.R.; Foster, D.G. Fundamentals of Momentum, Heat and Mass Transfer, 6th ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001; ISBN 978-0470504819. [Google Scholar]
- Hsu, C.H.; Li, M.H. Densities of Aqueous Blended Amines. J. Chem. Eng. Data 1997, 42, 502–507. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]












| Parameter | Value |
|---|---|
| Temperature, T (°C) | 25 |
| pH (-) | 7.0 ± 0.3 |
| Total organic carbon, TOC (mg L−1) | 2.2 ± 0.5 |
| Chemical oxygen demand, COD (mg L−1) | 18 ± 4 |
| Total solids, TS (mg L−1) | 1.7 ± 0.2 |
| Volatile solids, VS (mg L−1) | 1.3 ± 0.2 |
| Dissolved methane, dCH4 (mg L−1) | 11 ± 1 |
| Dissolved carbon dioxide, dCO2 (mg L−1) | 50 ± 10 |
| Parameter | Value |
|---|---|
| Membrane Contactor | |
| Membrane material | Polypropylene |
| Fiber inner diameter, di (m) | 2.20 × 10−4 |
| Fiber outer diameter, do (m) | 3.00 × 10−4 |
| Membrane thickness, δ (m) | 0.4 × 10−4 |
| Effective length of the fiber, L (m) | 0.1132 |
| Number of fibers, N | 7400 |
| Membrane pore diameter, dp (m) | 4.00 × 10−8 |
| Effective inner membrane area, Ai (m2) | 0.58 |
| Effective outer membrane area, Ao (m2) | 0.79 |
| Lumen side volume, Vl (mL) | 53 |
| Shell side volume, Vg (mL) | 78 |
| Porosity, ε | 40 |
| Packing factor, ϕ | 0.36 |
| Tortuosity, τ a | 6.4 |
| Operating conditions | |
| Operating configuration | Countercurrent |
| Operating temperature T (°C) | 25 |
| Liquid flowrate, Ql (mL min−1) | 100–400 |
| Liquid velocity, Vl (m s−1) | 5.93 × 10−3–2.37 × 10−2 |
| Liquid Reynold number, Rel (-) | 1.29–5.19 |
| Gas flowrate, Qg (mL min−1) | 50–200 |
| Gas velocity, Vg (m s−1) | 1.11 × 10−3–4.43 × 10 −3 |
| Gas Reynold number, Reg (-) | 3.70 × 10−2–1.48 × 10−1 |
| Reference | Effluent | Membrane | Operating Mode | Vl (m s−1) | Ql (mL min−1) | Vg (m s−1) | Qg (mL min−1) | Degassing Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| This Work | Saturated water | PP | Sweep gas | 0.006 | 100 | 0.0020 | 100 | 95.7 |
| This Work | Saturated water | PP | Sweep gas | 0.024 | 400 | 0.0020 | 100 | 78.1 |
| This Work | Saturated water | PP | Sweep gas | 0.006 | 100 | 0.004 | 200 | 78.1 |
| [36] | AnMBR prototype-plant | PDMS | Vacuum | - | 833 | - | - | 45.0 |
| [36] | AnMBR prototype-plant | PDMS | Vacuum | - | 3333 | - | - | 18.0 |
| [37] | Saturated water | PP | Sweep gas + vacuum | 0.016 | 83 | 0.0004 | 8.3 | 84 |
| [37] | Saturated water | PP | Sweep gas + vacuum | 0.089 | 466 | 0.0004 | 8.3 | 42 |
| [43] | EGSB | PP | Sweep gas + vacuum | 0.013 | 68 | 0.0220 | 433 | 82 |
| [43] | EGSB | PP | Sweep gas + vacuum | 0.086 | 453 | 0.0220 | 433 | 45 |
| Ql (mL min−1)/Vl (m s−1)/Rel | 100/0.006/1.30 | 400/0.024/5.19 |
|---|---|---|
| Qg (mL min−1)/Vg (m s−1)/Reg | Mix CH4 Flux (mg m−2 min−1) | |
| 50/0.001/0.04 | 1.47 | - |
| 100/0.002/0.07 | 1.71 | 5.27 |
| 200/0.004/0.15 | 1.93 | - |
| Pure CH4 Flux (mg m−2 min−1) | ||
| 100/0.002/0.07 | 3.77 | 11.07 |
| Ref. | Effluent | Membrane | Operating Mode | Vl (m s−1) | Ql (mL min−1) | Vg (m s−1) | Qg (mL min−1) | N (mg m−2 min−1) |
|---|---|---|---|---|---|---|---|---|
| This Work | Saturated water | PP | Sweep gas | 0.024 | 400 | 0.0020 | 100 | 11.07 |
| [36] | AnMBR prototype-plant | PDMS | Vacuum | - | 833 | - | - | 2.41 |
| [32] | Saturated water | PP | Sweep gas | 0.1 | - | - | 20 | 9.63 |
| [32] | Saturated water | Modified PVDF | Sweep gas | 0.1 | - | - | 20 | 18.28 |
| Ref. | Effluent | Membrane | Operating Mode | Vl (m s−1) | Ql (mL min−1) | Vg (m s−1) | Qg (mL min−1) | Kexp × 10 5 (m s−1) |
|---|---|---|---|---|---|---|---|---|
| This Work | Saturated water | PP | Sweep gas | 0.017 | 300 | 0.0020 | 100 | 1.93 |
| [37] | Saturated water | PP | Sweep gas + vacuum | 0.016 | 83 | 0.0004 | 8.3 | 1.65 |
| [36] | AnMBR prototype-plant | PDMS | Vacuum | - | 833 | - | 433- | 0.38 |
| [43] | EGSB | PP | Sweep gas + vacuum | 0.013 | 68 | 0.0220 | 1.94 | |
| [49] | Saturated water | PP | Sweep gas | 0.012 | 203 | 0.045 | - | 1.56 |
| [51] | Saturated water | PP | Sweep gas | 0.118 | 2000 | 0.018 | 1000 | 1.2 |
| [32] | Saturated water | PP | Sweep gas | 0.1 | - | - | 20 | 1.5 |
| [32] | Saturated water | Modified PVDF | Sweep gas | 0.1 | - | - | 20 | 2.5 |
| Property | Qg/Ql | Dm,g (m2 s−1) | Dm,eff (m2 s−1) | Kg (m s−1) | Kl (m s−1) | Km,nw (m s−1) | Km,w (m s−1) | Kov (m s−1) | Kexp (m s−1) |
|---|---|---|---|---|---|---|---|---|---|
| CH4 | 1 | 1.33 × 10−7 | 4.75 × 10−10 | 1.10 × 10−4 | 1.50 × 10−5 | 2.08 × 10−4 | 7.15 × 10−7 | 1.49 × 10−5 | 9.26 × 10−6 |
| CO2 | 1 | 2.20 × 10−7 | 2.79 × 10−9 | 8.40 × 10−5 | 1.54 × 10−5 | 3.44 × 10−4 | 4.36 × 10−6 | 1.35 × 10−5 | 4.31 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohaib, Q.; Kalakech, C.; Charmette, C.; Cartier, J.; Lesage, G.; Mericq, J.-P. Hollow-Fiber Membrane Contactor for Biogas Recovery from Real Anaerobic Membrane Bioreactor Permeate. Membranes 2022, 12, 112. https://doi.org/10.3390/membranes12020112
Sohaib Q, Kalakech C, Charmette C, Cartier J, Lesage G, Mericq J-P. Hollow-Fiber Membrane Contactor for Biogas Recovery from Real Anaerobic Membrane Bioreactor Permeate. Membranes. 2022; 12(2):112. https://doi.org/10.3390/membranes12020112
Chicago/Turabian StyleSohaib, Qazi, Carla Kalakech, Christophe Charmette, Jim Cartier, Geoffroy Lesage, and Jean-Pierre Mericq. 2022. "Hollow-Fiber Membrane Contactor for Biogas Recovery from Real Anaerobic Membrane Bioreactor Permeate" Membranes 12, no. 2: 112. https://doi.org/10.3390/membranes12020112
APA StyleSohaib, Q., Kalakech, C., Charmette, C., Cartier, J., Lesage, G., & Mericq, J. -P. (2022). Hollow-Fiber Membrane Contactor for Biogas Recovery from Real Anaerobic Membrane Bioreactor Permeate. Membranes, 12(2), 112. https://doi.org/10.3390/membranes12020112