Assessment of Graphical Methods for Determination of the Limiting Current Density in Complex Electrodialysis-Feed Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
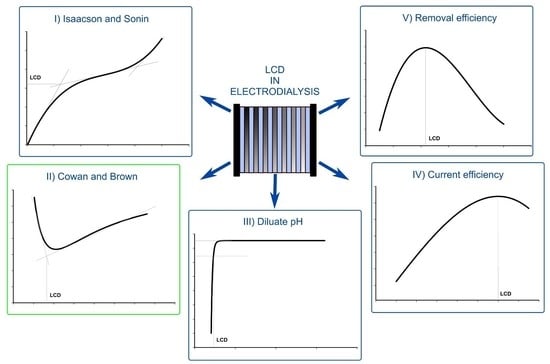
2.2. Description of Assessed LCD Determination Methods
2.2.1. Isaacson and Sonin (I–S) Method
2.2.2. Cowan and Brown (C–B) Method
2.2.3. The pH Method
2.2.4. Current Efficiency (λ) Method
2.2.5. Desalting Efficiency (ε) Method
2.3. Solution Tested
2.3.1. Solution Level a—Single-Salt Solution (SSS)
2.3.2. Solution Level b—Synthetic Complex Solution (SCS)
2.3.3. Solution Level c—Real Complex Solution (RCS)—Real Liquid Waste Streams
2.4. Data Processing
3. Results and Discussion
3.1. SSS—Range of NaCl Concentrations
3.2. Experiments with Different Ionic Compositions but the Same Conductivity
3.3. Same Ionic Composition but Different Initial Conductivity
4. Summary
5. Conclusions
- All of the assessed graphical methods showed LCDs for the feed concentrations ≤0.03 M NaCl. The current efficiency (λ) method did not have a clear peak at the LCD as defined by the method. Only the pH method revealed LCDs for feed concentrations ≥0.3 M NaCl.
- Four out of five LCD methods were applicable to each type of feed with increasing complexity. The current efficiency (λ) method was excluded, and the pH method was modified (±0.2 pH units from the initial feed pH). The C–B method had the most consistent results for all of the different types of feed solutions, and lower LCD values compared to the I–S method. In conclusion, the usage of the C–B method is highly recommended due to its applicability to a wide range of solutions with varying ionic compositions, concentrations, and matrix complexities.
- Solutions with the same conductivity but different ionic compositions do not reach the same LCD, as it is strongly influenced by the type and concentration of ionic species, and the presence of uncharged compounds.
- In one solution matrix, LCD linearly correlates with the decreasing conductivity. Therefore, the online conductivity measurement could be used for the batch ED control of one specific medium with a known composition. Otherwise, measuring only the conductivity is not suitable for current density adjustment unless combined with other characteristics of the feed solution.
- The obtained results bring knowledge to the existing graphical LCD methods and the complex medias’ behavior during electrodialysis membrane separation processes. The conclusions obtained in this work are useful for the selection of an appropriate LCD method in real feed solutions, LCD assessment, and future automatization of the electrodialysis.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Method by Isaacson and Sonin

Appendix A.2. Method by Cowan and Brown

Appendix A.3. The pH Method

Appendix A.4. Current Efficiency Method

Appendix A.5. Desalting Efficiency Method

References
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- van der Bruggen, B.; Milis, R.; Vandecasteele, C.; Bielen; van San, E.; Huysman, K. Electrodialysis and nanofiltration of surface water for subsequent use as infiltration water. Water Res. 2003, 37, 3867–3874. [Google Scholar] [CrossRef]
- Xu, T.; Huang, C. Electrodialysis-based separation technologies: A critical review. AIChE J. 2008, 54, 3147–3159. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Mohammadi, R.; Tang, W.; Sillanpää, M. A systematic review and statistical analysis of nutrient recovery from municipal wastewater by electrodialysis. Desalination 2021, 498, 114626. [Google Scholar] [CrossRef]
- Ward, A.J.; Arola, K.; Brewster, E.T.; Mehta, C.M.; Batstone, D.J. Nutrient recovery from wastewater through pilot scale electrodialysis. Water Res. 2018, 135, 57–65. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Al-Karaghouli, A.; Kazmerski, L.L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. [Google Scholar] [CrossRef]
- Sosa-Fernandez, P.A.; Post, J.W.; Ramdlan, M.S.; Leermakers, F.A.M.; Bruning, H.; Rijnaarts, H.H.M. Improving the performance of polymer-flooding produced water electrodialysis through the application of pulsed electric field. Desalination 2020, 484, 114424. [Google Scholar] [CrossRef]
- Tanaka, Y. Mass transport and energy consumption in ion-exchange membrane electrodialysis of seawater. J. Membr. Sci. 2003, 215, 265–279. [Google Scholar] [CrossRef]
- Wu, D.; Chen, G.Q.; Hu, B.; Deng, H. Feasibility and energy consumption analysis of phenol removal from salty wastewater by electro-electrodialysis. Sep. Purif. Technol. 2019, 215, 44–50. [Google Scholar] [CrossRef]
- Ankoliya, D.; Mudgal, A.; Sinha, M.K.; Davies, P.; Licon, E.; Alegre, R.R.; Patel, V.; Patel, J. Design and optimization of electrodialysis process parameters for brackish water treatment. J. Clean. Prod. 2021, 319, 128686. [Google Scholar] [CrossRef]
- Generous, M.M.; Qasem, N.A.A.; Akbar, U.A.; Zubair, S.M. Techno-economic assessment of electrodialysis and reverse osmosis desalination plants. Sep. Purif. Technol. 2021, 272, 118875. [Google Scholar] [CrossRef]
- Kucera, J. (Ed.) Desalination: Water from Water; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Lee, H.-J.; Strathmann, H.; Moon, S.-H. Determination of the limiting current density in electrodialysis desalination as an empirical function of linear velocity. Desalination 2006, 190, 43–50. [Google Scholar] [CrossRef]
- Li, F.; Jia, Y.; He, J.; Wang, M. Effects of electrodialysis conditions on waste acid reclamation: Modelling and validation. J. Clean. Prod. 2021, 320, 128760. [Google Scholar] [CrossRef]
- Strathmann, H. Chapter 6 Electrodialysis and related processes. In Membrane Science and Technology, 2nd ed.; Noble, R.D., Stern, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 213–281. [Google Scholar] [CrossRef]
- La Cerva, M.F.; Gurreri, L.; Tedesco, M.; Cipollina, A.; Ciofalo, M.; Tamburini, A.; Micale, G. Determination of limiting current density and current efficiency in electrodialysis units. Desalination 2018, 445, 138–148. [Google Scholar] [CrossRef]
- Nakayama, A.; Sano, Y.; Bai, X.; Tado, K. A boundary layer analysis for determination of the limiting current density in an electrodialysis desalination. Desalination 2017, 404, 41–49. [Google Scholar] [CrossRef]
- Nikonenko, V.; Kovalenko, A.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Pawlowski, S.; Sistat, P.; Crespo, J.G.; Velizarov, S. Mass transfer in reverse electrodialysis: Flow entrance effects and diffusion boundary layer thickness. J. Membr. Sci. 2014, 471, 72–83. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Doyen, A.; Roblet, C.; L’Archevêque-Gaudet, A.; Bazinet, L. Mathematical sigmoid-model approach for the determination of limiting and over-limiting current density values. J. Membr. Sci. 2014, 452, 453–459. [Google Scholar] [CrossRef]
- Rösler, H.-W.; Maletzki, F.; Staude, E. Ion transfer across electrodialysis membranes in the overlimiting current range: Chronopotentiometric studies. J. Membr. Sci. 1992, 72, 171–179. [Google Scholar] [CrossRef]
- Rubinstein, I. Electroconvection at an electrically inhomogeneous permselective interface. Phys. Fluids A: Fluid Dyn. 1991, 3, 2301–2309. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Urtenov, M.K. Coupled transport phenomena in overlimiting current electrodialysis. Sep. Purif. Technol. 1998, 14, 255–267. [Google Scholar] [CrossRef]
- Benneker, A.M.; Klomp, J.; Lammertink, R.G.H.; Wood, J.A. Influence of temperature gradients on mono- and divalent ion transport in electrodialysis at limiting currents. Desalination 2018, 443, 62–69. [Google Scholar] [CrossRef]
- Mahendra, C.; Sai, P.M.S.; Babu, C.A. Current–voltage characteristics in a hybrid electrodialysis–ion exchange system for the recovery of cesium ions from ammonium molybdophosphate-polyacrylonitrile. Desalination 2014, 353, 8–14. [Google Scholar] [CrossRef]
- Min, K.J.; Kim, J.H.; Park, K.Y. Characteristics of heavy metal separation and determination of limiting current density in a pilot-scale electrodialysis process for plating wastewater treatment. Sci. Total Environ. 2021, 757, 143762. [Google Scholar] [CrossRef]
- Xutoi, S.; Guang, C. The calculation of limiting current density of electrodialysis under the influence of water quality and temperature. Desalination 1983, 46, 263–274. [Google Scholar] [CrossRef]
- van Linden, N.; Spanjers, H.; van Lier, J.B. Application of dynamic current density for increased concentration factors and reduced energy consumption for concentrating ammonium by electrodialysis. Water Res. 2019, 163, 114856. [Google Scholar] [CrossRef]
- He, W.; le Henaff, A.-C.; Amrose, S.; Buonassisi, T.; Peters, I.M.; Winter, A.G. Voltage- and flow-controlled electrodialysis batch operation: Flexible and optimized brackish water desalination. Desalination 2021, 500, 114837. [Google Scholar] [CrossRef]
- Bittencourt, S.D.; Marder, L.; Benvenuti, T.; Ferreira, J.Z.; Bernardes, A.M. Analysis of different current density conditions in the electrodialysis of zinc electroplating process solution. Sep. Sci. Technol. 2017, 52, 2079–2089. [Google Scholar] [CrossRef]
- Cowan, D.A.; Brown, J.H. Effect of Turbulence on Limiting Current in Electrodialysis Cells. Ind. Eng. Chem. 1959, 51, 1445–1448. [Google Scholar] [CrossRef]
- Meng, H.; Deng, D.; Chen, S.; Zhang, G. A new method to determine the optimal operating current (Ilim’) in the electrodialysis process. Desalination 2005, 181, 101–108. [Google Scholar] [CrossRef]
- Isaacson, M.S.; Sonin, A.A. Sherwood Number and Friction Factor Correlations for Electrodialysis Systems, with Application to Process Optimization. Ind. Eng. Chem. Proc. Des. Dev. 1976, 15, 313–321. [Google Scholar] [CrossRef]
- Melin, T.; Rautenbach, R. Membranverfahren: Grundlagen der Modul-und Anlagenauslegung, 3th ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Rubinstein, I.; Shtilman, L. Voltage against current curves of cation exchange membranes. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1979, 75, 231–246. [Google Scholar] [CrossRef]
- Valerdi-Pérez, R.; Ibáñez-Mengual, J. Current—voltage curves for an electrodialysis reversal pilot plant: Determination of limiting currents. Desalination 2001, 141, 23–37. [Google Scholar] [CrossRef]
- Krol, J.J.; Wessling, M.; Strathmann, H. Chronopotentiometry and overlimiting ion transport through monopolar ion exchange membranes. J. Membr. Sci. 1999, 162, 155–164. [Google Scholar] [CrossRef]
- Kwak, R.; Guan, G.; Peng, W.K.; Han, J. Microscale electrodialysis: Concentration profiling and vortex visualization. Desalination 2013, 308, 138–146. [Google Scholar] [CrossRef]
- Dortwegt, R.; Maughan, E.V. The chemistry of copper in water and related studies planned at the Advanced Photon Source. In Proceedings of the 2001 Particle Accelerator Conference (Cat. No.01CH37268), Chicago, IL, USA, 18–22 June 2001; Volume 2, pp. 1456–1458. [Google Scholar] [CrossRef] [Green Version]
- Daza-Serna, L.; Knežević, K.; Kreuzinger, N.; Mach-Aigner, A.R.; Mach, R.L.; Krampe, J.; Friedl, A. Recovery of Salts from Synthetic Erythritol Culture Broth via Electrodialysis: An Alternative Strategy from the Bin to the Loop. Sustainability 2022, 14, 734. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Jensen, S.M.; Fricker, G.; Brandl, M.; Treusch, A.H. Archaeal lipids in oral delivery of therapeutic peptides. Eur. J. Pharm. Sci. 2017, 108, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Quehenberger, J.; Albersmeier, A.; Glatzel, H.; Hackl, M.; Kalinowski, J.; Spadiut, O. A defined cultivation medium for Sulfolobus acidocaldarius. J. Biotechnol. 2019, 301, 56–67. [Google Scholar] [CrossRef]
- Quehenberger, J.; Shen, L.; Albers, S.-V.; Siebers, B.; Spadiut, O. Sulfolobus—A Potential Key Organism in Future Biotechnology. Front. Microbiol. 2017, 8, 2474. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Huang, S.; Wang, J.; Zhang, X. Limiting concentration during batch electrodialysis process for concentrating high salinity solutions: A theoretical and experimental study. Desalination 2021, 498, 114793. [Google Scholar] [CrossRef]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electroconvective instability. J. Fluid Mech. 2007, 579, 173–226. [Google Scholar] [CrossRef]
- Pismenskaya, N.D.; Nikonenko, V.; Belova, E.I.; Lopatkova, G.Y.; Sistat, P.; Pourcelly, G.; Larshe, K. Coupled convection of solution near the surface of ion-exchange membranes in intensive current regimes. Russ. J. Electrochem. 2007, 43, 307–327. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Electro-osmotically induced convection at a permselective membrane. Phys. Rev. E 2000, 62, 2238–2251. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Huang, S.; Su, W.; Zhou, J.; Zhang, X. Performance evaluation on regeneration of high-salt solutions used in air conditioning systems by electrodialysis. J. Membr. Sci. 2019, 582, 224–235. [Google Scholar] [CrossRef]
- Długołęcki, P.; Anet, B.; Metz, S.J.; Nijmeijer, K.; Wessling, M. Transport limitations in ion exchange membranes at low salt concentrations. J. Membr. Sci. 2010, 346, 163–171. [Google Scholar] [CrossRef]
- Kooistra, W. Characterization of ion exchange membranes by polarization curves. Desalination 1967, 2, 139–147. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T. Treatment of sea water using electrodialysis: Current efficiency evaluation. Desalination 2009, 249, 279–285. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Zhou, Y.; Irfan, M.; Wang, Y.; Jiang, C.; Xu, T. In-Situ Combination of Bipolar Membrane Electrodialysis with Monovalent Selective Anion-Exchange Membrane for the Valorization of Mixed Salts into Relatively High-Purity Monoprotic and Diprotic Acids. Membranes 2020, 10, 135. [Google Scholar] [CrossRef]
- Geraldes, V.; Afonso, M.D. Limiting current density in the electrodialysis of multi-ionic solutions. J. Membr. Sci. 2010, 360, 499–508. [Google Scholar] [CrossRef]
- Galama, A.H.; Daubaras, G.; Burheim, O.S.; Rijnaarts, H.H.M.; Post, J.W. Seawater electrodialysis with preferential removal of divalent ions. J. Membr. Sci. 2014, 452, 219–228. [Google Scholar] [CrossRef]
- Lu, C.; Tahir, N.; Li, W.; Zhang, Z.; Jiang, D.; Guo, S.; Wang, J.; Wang, K.; Zhang, Q. Enhanced buffer capacity of fermentation broth and biohydrogen production from corn stalk with Na2HPO4/NaH2PO4. Bioresour. Technol. 2020, 313, 123783. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology, 3th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]











| Membrane | Type | Thickness, µm | Transference Number | Resistance, Ohm cm2 | Water Content (wt%) | pH Stability |
|---|---|---|---|---|---|---|
| PC SA | Anion-exchange | 100–110 | >0.95 | 1.8 | 14 | 0–9 |
| PC SK | Cation-exchange | 100–120 | >0.95 | 2.5 | 9 | 0–11 |
| PC MTE | End membrane | 220 | >0.94 | 4.5 | - | 1–13 |
| Solution | TOC | PO4-P | Cl− | SO42− | NH4-N | Na+ | K+ | Ca2+ | Mg2+ | Conductivity |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | mmol/L | mS/cm | ||||||||
| SSS1 | 0 | 0 | 53.6 | 0 | 0 | 53.8 | 0 | 0 | 0 | 5.5 |
| SSS2 | 0 | 0 | 0 | 19.5 | 0 | 9.4 | 0 | 0 | 0 | 5.5 |
| SCS1 | 0 | 13.2 | 12.0 | 12.1 | 12.8 | 11.9 | 13.2 | 0 | 2.8 | 5.5 |
| SCS2 | 0 | 4.3 | 2.01 | 71.0 | 92.2 | 25.7 | 2.8 | 0.8 | 1.0 | 13.8 |
| RCS | 1060 | 4.1 | 2.2 | 85.1 | 94.4 | 37.5 | 4.7 | 1.3 | 2.5 | 18.4 |
| Solution | NaCl 1 | NaCl 2 | NaCl 5.5 | NaCl 30.6 | NaCl 48.7 |
|---|---|---|---|---|---|
| NaCl concentration [mol/L] | 0.005 | 0.004 | 0.03 | 0.3 | 0.5 |
| Conductivity [mS/cm] | 1 | 2 | 5.5 | 30.6 | 48.7 |
| Average temperature T [°C] | 19.1 | 19.2 | 20.2 | 20.4 | 21.0 |
| Initial pH | 5.16 | 5.23 | 5.21 | 5.20 | 4.80 |
| LCDs [A/m2] | |||||
| I–S | 30.1 | 58.8 | 175.5 | / | / |
| C–B | 23.5 | 41.7 | 130.9 | / | / |
| pH Method | 43.8 | 78.1 | 209.6 | 771.2 | / |
| Max pH * | 43.8 | 72.9 | 203.1 | 554.7 | 693.6 |
| pH decrease ** | 34.2 | 67.2 | 198.4 | 382.8 | 459.7 |
| λ Method *** | 43.7 | 67.6 | 107.8 | 108.0 | 78.1 |
| ε Method | 23.4 | 42.8 | 167.5 | / | / |
| Solution | SSS1 (NaCl) | SSS2 (Na2SO4) | SCS1 | SCS2 | RCS |
|---|---|---|---|---|---|
| Conductivity [mS/cm] | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| Average temperature [°C] | 20.2 | 22.5 | 23.5 | 21.5 | 21.1 |
| Initial pH | 6.31 | 8.49 | 4.59 | 3.24 | 2.25 |
| LCD [A/m2] | |||||
| I&S | 175.5 | 153.2 | 179.0 | 135.7 | 117.2 |
| C&B | 130.9 | 119.3 | 176.8 | 102.3 | 89.6 |
| pH method | 209.6 | 137.6 | 90.5 | (112.5) * | (76.5) * |
| ε Method | 167.5 | 158.3 | 137.4 | 118.0 | 112.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knežević, K.; Reif, D.; Harasek, M.; Krampe, J.; Kreuzinger, N. Assessment of Graphical Methods for Determination of the Limiting Current Density in Complex Electrodialysis-Feed Solutions. Membranes 2022, 12, 241. https://doi.org/10.3390/membranes12020241
Knežević K, Reif D, Harasek M, Krampe J, Kreuzinger N. Assessment of Graphical Methods for Determination of the Limiting Current Density in Complex Electrodialysis-Feed Solutions. Membranes. 2022; 12(2):241. https://doi.org/10.3390/membranes12020241
Chicago/Turabian StyleKnežević, Katarina, Daniela Reif, Michael Harasek, Jörg Krampe, and Norbert Kreuzinger. 2022. "Assessment of Graphical Methods for Determination of the Limiting Current Density in Complex Electrodialysis-Feed Solutions" Membranes 12, no. 2: 241. https://doi.org/10.3390/membranes12020241
APA StyleKnežević, K., Reif, D., Harasek, M., Krampe, J., & Kreuzinger, N. (2022). Assessment of Graphical Methods for Determination of the Limiting Current Density in Complex Electrodialysis-Feed Solutions. Membranes, 12(2), 241. https://doi.org/10.3390/membranes12020241