Surface Modification of Matrimid® 5218 Polyimide Membrane with Fluorine-Containing Diamines for Efficient Gas Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Matrimid Membrane
2.3. Diamine Modification of Matrimid Membrane
2.4. Characterization
2.5. Gas Permeation Measurement
3. Results and Discussion
3.1. Preparation and Characterization of Diamine-Modified Matrimid Membranes
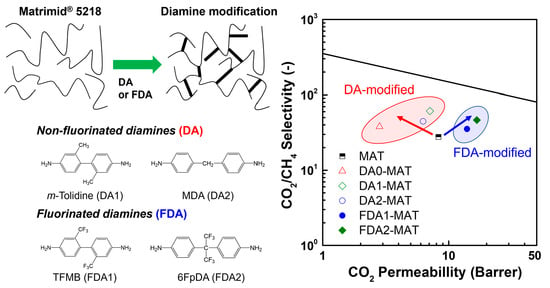
3.2. Gas Separation Performance of Diamine-Modified Matrimid Membranes
3.3. Explaining the Gas Transport Properties of Diamine-Modified Matrimid Membranes: The Role of Fluorine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Sanders, D.E.; Smith, Z.P.; Guo, R.L.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Efficient Carbon Resource Processing, Utilization, and Recycling towards Carbon Neutrality. Angew. Chem. Int. Ed. 2021, e202112835. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymeric Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Koros, W.J.; Lively, R.P. Water and beyond: Expanding the spectrum of large-scale energy efficient separation processes. AlChE J. 2012, 58, 2624–2633. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- White, L.S.; Blinka, T.A.; Kloczewski, H.A.; Wang, I.F. Properties of a Polyimide Gas Separation Membrane in Natural-Gas Streams. J. Membr. Sci. 1995, 103, 73–82. [Google Scholar] [CrossRef]
- Ayala, D.; Lozano, A.E.; de Abajo, J.; Garcia-Perez, C.; de la Campa, J.G.; Peinemann, K.V.; Freeman, B.D.; Prabhakar, R. Gas separation properties of aromatic polyimides. J. Membr. Sci. 2003, 215, 61–73. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, H.B. High Performance Polyimide with High Internal Free Volume Elements. Macromol. Rapid. Commun. 2011, 32, 579–586. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van der Bruggen, B. Polyimides in membrane gas separation: Monomer’s molecular design and structural engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Lee, T.H.; Ozcan, A.; Park, I.; Fan, D.; Jang, J.K.; Mileo, P.G.M.; Yoo, S.Y.; Roh, J.S.; Kang, J.H.; Lee, B.K.; et al. Disclosing the Role of Defect-Engineered Metal-Organic Frameworks in Mixed Matrix Membranes for Efficient CO2 Separation: A Joint Experimental-Computational Exploration. Adv. Funct. Mater. 2021, 31, 2103973. [Google Scholar] [CrossRef]
- Lee, T.H.; Oh, J.Y.; Jang, J.K.; Moghadam, F.; Roh, J.S.; Yoo, S.Y.; Kim, Y.J.; Choi, T.H.; Lin, H.; Kim, H.W.; et al. Elucidating the Role of Embedded Metal-Organic Frameworks in Water and Ion Transport Properties in Polymer Nanocomposite Membranes. Chem. Mater. 2020, 32, 10165–10175. [Google Scholar] [CrossRef]
- Lee, T.H.; Jung, J.G.; Kim, Y.J.; Roh, J.S.; Yoon, H.W.; Ghanem, B.S.; Kim, H.W.; Cho, Y.H.; Pinnau, I.; Park, H.B. Defect Engineering in Metal-Organic Frameworks Towards Advanced Mixed Matrix Membranes for Efficient Propylene/Propane Separation. Angew. Chem. Int. Ed. 2021, 60, 13081–13088. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.V. Pebax®/polyethylene glycol blend thin film composite membranes for CO2 separation: Performance with mixed gases. Sep. Purif. Technol. 2008, 62, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Moghadam, F.; Lee, T.H.; Park, I.; Park, H.B. Thermally annealed polyimide-based mixed matrix membrane containing ZIF-67 decorated porous graphene oxide nanosheets with enhanced propylene/propane selectivity. J. Membr. Sci. 2020, 603, 118019. [Google Scholar] [CrossRef]
- Park, H.B.; Han, D.W.; Lee, Y.M. Effect of a UV/ozone treatment on siloxane-containing copolyimides: Surface modification and gas transport characteristics. Chem. Mater. 2003, 15, 2346–2353. [Google Scholar] [CrossRef]
- Park, J.; Gaines, K.E.; Jheng, L.C.; Riffle, J.S.; Mecham, S.J.; McGrath, J.E.; Park, H.B.; Paul, D.R.; Freeman, B.D. Characterization and gas transport properties of UV-irradiated polydimethylsiloxane (PDMS)-containing polyimide copolymer membranes. Polymer 2020, 210, 122966. [Google Scholar] [CrossRef]
- Lee, T.H.; Park, I.; Oh, J.Y.; Jang, J.K.; Park, H.B. Facile Preparation of Polyamide Thin-Film Nanocomposite Membranes Using Spray-Assisted Nanofiller Predeposition. Ind. Eng. Chem. Res. 2019, 58, 4248–4256. [Google Scholar] [CrossRef]
- Zhang, M.L.; Deng, L.M.; Xiang, D.X.; Cao, B.; Hosseini, S.S.; Li, P. Approaches to Suppress CO2-Induced Plasticization of Polyimide Membranes in Gas Separation Applications. Processes 2019, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Pal, S.; Nguyen, H.; Bui, V.; Lin, H. Molecularly engineering polymeric membranes for H2/CO2 separation at 100–300 °C. J. Polym. Sci. 2020, 58, 2467–2481. [Google Scholar] [CrossRef]
- Tin, P.S.; Chung, T.S.; Liu, Y.; Wang, R.; Liu, S.L.; Pramoda, K.P. Effects of cross-linking modification on gas separation performance of Matrimid membranes. J. Membr. Sci. 2003, 225, 77–90. [Google Scholar] [CrossRef]
- Liu, Y.; Chung, T.S.; Wang, R.; Li, D.F.; Chng, M.L. Chemical cross-linking modification of polyimide/poly(ether sulfone) dual-layer hollow-fiber membranes for gas separation. Ind. Eng. Chem. Res. 2003, 42, 1190–1195. [Google Scholar] [CrossRef]
- Shao, L.; Chung, T.S.; Goh, S.H.; Pramoda, K.P. Transport properties of cross-linked polyimide membranes induced by different generations of diaminobutane (DAB) dendrimers. J. Membr. Sci. 2004, 238, 153–163. [Google Scholar] [CrossRef]
- Shao, L.; Chung, T.S.; Goh, S.H.; Pramoda, K.P. The effects of 1,3-cyclohexanebis(methylamine) modification on gas transport and plasticization resistance of polyimide membranes. J. Membr. Sci. 2005, 267, 78–89. [Google Scholar] [CrossRef]
- Shao, L.; Chung, T.S.; Goh, S.H.; Pramoda, K.P. Polyimide modification by a linear aliphatic diamine to enhance transport performance and plasticization resistance. J. Membr. Sci. 2005, 256, 46–56. [Google Scholar] [CrossRef]
- Shao, L.; Liu, L.; Cheng, S.X.; Huang, Y.D.; Ma, J. Comparison of diamino cross-linking in different polyimide solutions and membranes by precipitation observation and gas transport. J. Membr. Sci. 2008, 312, 174–185. [Google Scholar] [CrossRef]
- Wu, A.X.; Drayton, J.A.; Smith, Z.P. The perfluoropolymer upper bound. AlChE J. 2019, 65, e16700. [Google Scholar] [CrossRef]
- Yampolskii, Y.P.; Belov, N.A.; Alentiev, A.Y. Fluorine in the structure of polymers: Influence on the gas separation properties. Russ. Chem. Rev. 2019, 88, 387–405. [Google Scholar] [CrossRef]
- Wu, A.X.; Drayton, J.A.; Rodriguez, K.M.; Benedetti, F.M.; Qian, Q.H.; Lin, S.R.; Smith, Z.P. Elucidating the Role of Fluorine Content on Gas Sorption Properties of Fluorinated Polyimides. Macromolecules 2021, 54, 22–34. [Google Scholar] [CrossRef]
- Wu, A.X.; Drayton, J.A.; Rodriguez, K.M.; Qian, Q.H.; Lin, S.R.; Smith, Z.P. Influence of Aliphatic and Aromatic Fluorine Groups on Gas Permeability and Morphology of Fluorinated Polyimide Films. Macromolecules 2020, 53, 5085–5095. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Martin-Gil, V.; Ahmad, M.Z.; Fila, V. Matrimid® 5218 in preparation of membranes for gas separation: Current state-of-the-art. Chem. Eng. Commun. 2018, 205, 161–196. [Google Scholar] [CrossRef]
- Omidvar, M.; Stafford, C.M.; Lin, H. Thermally stable cross-linked P84 with superior membrane H2/CO2 separation properties at 100 °C. J. Membr. Sci. 2019, 575, 118–125. [Google Scholar] [CrossRef]
- Kim, S.J.; Ahn, Y.; Kim, J.F.; Nam, S.E.; Park, H.; Cho, Y.H.; Baek, K.Y.; Park, Y.I. Comparison of liquid-phase and methanol-swelling crosslinking processes of polyimide dense membrane for CO2/CH4 separation. J. Appl. Polym. Sci. 2021, 138, 7. [Google Scholar] [CrossRef]
- Kim, S.J.; Ahn, Y.; Kim, J.F.; Nam, S.E.; Park, H.; Cho, Y.H.; Baek, K.Y.; Park, Y.I. Effect of solvent on crosslinking of a polyimide membrane using the liquid-phase crosslinking process for CO2/CH4 separation. Sep. Purif. Technol. 2021, 260, 118213. [Google Scholar] [CrossRef]
- Lee, T.H.; Oh, J.Y.; Hong, S.P.; Lee, J.M.; Roh, S.M.; Kim, S.H.; Park, H.B. ZIF-8 particle size effects on reverse osmosis performance of polyamide thin-film nanocomposite membranes: Importance of particle deposition. J. Membr. Sci. 2019, 570, 23–33. [Google Scholar] [CrossRef]
- Zhang, L.L.; Fang, W.J.; Jiang, J.W. Effects of Residual Solvent on Membrane Structure and Gas Permeation in a Polymer of Intrinsic Microporosity: Insight from Atomistic Simulation. J. Phys. Chem. C 2011, 115, 11233–11239. [Google Scholar] [CrossRef]
- Zuo, H.T.; Gan, F.; Dong, J.; Zhang, P.; Zhao, X.; Zhang, Q.H. Highly Transparent and Colorless Polyimide Film with Low Dielectric Constant by Introducing Meta-substituted Structure and Trifluoromethyl Groups. Chin. J. Polym. Sci. 2021, 39, 455–464. [Google Scholar] [CrossRef]
- Ghaemy, M.; Berenjestanaki, F.R.; Bazzar, M. Organosoluble, thermally stable and low dielectric constant fluorinated polyimides containing 2,4,5-triphenylimidazole moiety in the main chains. Des. Monomers Polym. 2014, 17, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Yerzhankyzy, A.; Wang, Y.G.; Ghanem, B.S.; Puspasari, T.; Pinnau, I. Gas separation performance of solid-state in-situ thermally crosslinked 6FDA-based polyimides. J. Membr. Sci. 2022, 641, 119885. [Google Scholar] [CrossRef]
- Lee, T.H.; Moghadam, F.; Jung, J.G.; Kim, Y.J.; Roh, J.S.; Yoo, S.Y.; Lee, B.K.; Kim, J.H.; Pinnau, I.; Park, H.B. In Situ Derived Hybrid Carbon Molecular Sieve Membranes with Tailored Ultramicroporosity for Efficient Gas Separation. Small 2021, 17, 2104698. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Pelletier, H.; Martin-Gil, V.; Castro-Munoz, R.; Fila, V. Chemical Crosslinking of 6FDA-ODA and 6FDA-ODA:DABA for Improved CO2/CH4 Separation. Membranes 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yang, Y.T.; Ho, W.S.W. Recent Progress in the Engineering of Polymeric Membranes for CO2 Capture from Flue Gas. Membranes 2020, 10, 365. [Google Scholar] [CrossRef]
- Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. Hydrogen Separation and Purification from Various Gas Mixtures by Means of Electrochemical Membrane Technology in the Temperature Range 100–160 °C. Membranes 2021, 11, 282. [Google Scholar] [CrossRef]
- Park, S.C.; Lee, T.H.; Moon, G.H.; Kim, B.S.; Roh, J.M.; Cho, Y.H.; Kim, H.W.; Jang, J.; Park, H.B.; Kang, Y.S. Sub-5 nm Graphene Oxide Nanofilm with Exceptionally High H+/V Selectivity for Vanadium Redox Flow Battery. ACS Appl. Energy Mater. 2019, 2, 4590–4596. [Google Scholar] [CrossRef]
- Roh, J.S.; Choi, T.H.; Lee, T.H.; Yoon, H.W.; Kim, J.; Kim, H.W.; Park, H.B. Understanding Gas Transport Behavior through Few-Layer Graphene Oxide Membranes Controlled by Tortuosity and Interlayer Spacing. J. Phys. Chem. Lett. 2019, 10, 7725–7731. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.B.; Seong, J.G.; Do, Y.S.; Jo, H.J.; Lee, M.J.; Wang, G.; Guiver, M.D.; Lee, Y.M. Effect of Isomerism on Molecular Packing and Gas Transport Properties of Poly(benzoxazole-co-imide)s. Macromolecules 2014, 47, 7947–7957. [Google Scholar] [CrossRef]
- Lee, T.H.; Shin, M.G.; Jung, J.G.; Suh, E.H.; Oh, J.G.; Kang, J.H.; Ghanem, B.S.; Jang, J.; Lee, J.-H.; Pinnau, I.; et al. Facile suppression of intensified plasticization in glassy polymer thin films towards scalable composite membranes for propylene/propane separation. J. Membr. Sci. 2022, 645, 120215. [Google Scholar] [CrossRef]
- Du, N.Y.; Dal-Cin, M.M.; Pinnau, I.; Nicalek, A.; Robertson, G.P.; Guiver, M.D. Azide-based Cross-Linking of Polymers of Intrinsic Microporosity (PIMs) for Condensable Gas Separation. Macromol. Rapid. Commun. 2011, 32, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Borova, S.; Tokarev, V.; Stahlhut, P.; Luxenhofer, R. Crosslinking of hydrophilic polymers using polyperoxides. Colloid Polym. Sci. 2020, 298, 1699–1713. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H. Crosslinked poly(methyl methacrylate) with perfluorocyclobutyl aryl ether moiety as crosslinking unit: Thermally stable polymer with high glass transition temperature. RSC Adv. 2020, 10, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- Low, B.T.; Chung, T.S.; Chen, H.; Jean, Y.-C.; Pramoda, K.P. Tuning the Free Volume Cavities of Polyimide Membranes via the Construction of Pseudo-Interpenetrating Networks for Enhanced Gas Separation Performance. Macromolecules 2009, 42, 7042–7054. [Google Scholar] [CrossRef]
- Low, Z.X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef]







| Membranes | Atomic Concentration (at.%) | |||
|---|---|---|---|---|
| C | N | O | F | |
| Mat | 81.44 | 4.13 | 14.43 | - |
| FDA1-MAT | 77.83 | 4.11 | 13.95 | 4.11 |
| FDA2-MAT | 81.08 | 4.09 | 12.35 | 2.48 |
| Gas | Gas Permeability (Barrer) | |||||
|---|---|---|---|---|---|---|
| MAT | DA0-MAT | DA1-MAT | DA2-MAT | FDA1-MAT | FDA2-MAT | |
| H2 | 21.6 | 13.9 | 23.1 | 23.3 | 47.0 | 41.0 |
| CO2 | 8.3 | 2.8 | 7.1 | 6.3 | 14.0 | 16.7 |
| O2 | 1.9 | 0.9 | 1.4 | 1.6 | 3.1 | 3.4 |
| N2 | 0.3 | 0.09 | 0.2 | 0.2 | 0.5 | 0.5 |
| CH4 | 0.3 | 0.07 | 0.1 | 0.1 | 0.4 | 0.3 |
| Gas pair | Selectivity (-) | |||||
| H2/N2 | 72 | 152 | 105 | 117 | 98 | 77 |
| H2/CH4 | 79 | 186 | 199 | 166 | 119 | 114 |
| O2/N2 | 6.3 | 9.3 | 6.5 | 7.9 | 6.5 | 6.5 |
| CO2/N2 | 28 | 31 | 32 | 31 | 29 | 32 |
| CO2/CH4 | 31 | 38 | 61 | 45 | 35 | 46 |
| Polyimide | Modifier | CO2 Permeability (Barrer) | CO2/CH4 Selectivity (-) | CO2 Permeability Enhancement (%) | CO2/CH4 Selectivity Enhancement (%) | Ref. |
|---|---|---|---|---|---|---|
| Matrimid | p-XDA | 1.9 | 28 | −71 | −18 | [26] |
| Matrimid | EDA | 1.2 | 27.7 | −81 | −19 | [31] |
| Matrimid | BuDA | 2.1 | 18.7 | −68 | −45 | [31] |
| Matrimid | p-PDA | 4.2 | 46.1 | −58 | +15 | [38] |
| Matrimid | p-PDA | 9.5 | 45.9 | −6 | +14 | [39] |
| 6FDA-durene | DABD | 244.8 | 23.6 | −60 | +74 | [28] |
| 6FDA-durene | CHBA | 55.2 | 19.3 | −91 | +42 | [29] |
| 6FDA-durene | EDA | 21.2 | 22.3 | −97 | +64 | [30] |
| 6FDA-durene | EDA | 435 | 21.1 | −29 | +55 | [31] |
| 6FDA-durene | PDA | 81.1 | 27.8 | −87 | +104 | [31] |
| 6FDA-durene | BuDA | 218 | 24.7 | −64 | +82 | [31] |
| 6FDA-ODA | p-XDA | 10.6 | 58.8 | −76 | +97 | [47] |
| 6FDA-ODA | n-ethylamine | 7.8 | 37.5 | −82 | +25 | [47] |
| 6FDA-ODA | n-butylamine | 8.8 | 42.9 | −80 | +43 | [47] |
| Matrimid | DA1 | 7.1 | 61 | −14 | +97 | This work |
| Matrimid | DA2 | 6.3 | 45 | −24 | +45 | |
| Matrimid | FDA1 | 14 | 35 | +69 | +13 | |
| Matrimid | FDA2 | 16.7 | 46 | +101 | +48 |
| Membranes | Density (g/cm3) |
|---|---|
| MAT | 1.242 ± 0.008 |
| DA0-MAT | 1.265 ± 0.009 |
| DA1-MAT | 1.248 ± 0.012 |
| DA2-MAT | 1.252 ± 0.009 |
| FDA1-MAT | 1.245 ± 0.009 |
| FDA2-MAT | 1.240 ± 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.H.; Lee, B.K.; Park, J.S.; Park, J.; Kang, J.H.; Yoo, S.Y.; Park, I.; Kim, Y.-H.; Park, H.B. Surface Modification of Matrimid® 5218 Polyimide Membrane with Fluorine-Containing Diamines for Efficient Gas Separation. Membranes 2022, 12, 256. https://doi.org/10.3390/membranes12030256
Lee TH, Lee BK, Park JS, Park J, Kang JH, Yoo SY, Park I, Kim Y-H, Park HB. Surface Modification of Matrimid® 5218 Polyimide Membrane with Fluorine-Containing Diamines for Efficient Gas Separation. Membranes. 2022; 12(3):256. https://doi.org/10.3390/membranes12030256
Chicago/Turabian StyleLee, Tae Hoon, Byung Kwan Lee, Jin Sung Park, Jinmo Park, Jun Hyeok Kang, Seung Yeon Yoo, Inho Park, Yo-Han Kim, and Ho Bum Park. 2022. "Surface Modification of Matrimid® 5218 Polyimide Membrane with Fluorine-Containing Diamines for Efficient Gas Separation" Membranes 12, no. 3: 256. https://doi.org/10.3390/membranes12030256
APA StyleLee, T. H., Lee, B. K., Park, J. S., Park, J., Kang, J. H., Yoo, S. Y., Park, I., Kim, Y. -H., & Park, H. B. (2022). Surface Modification of Matrimid® 5218 Polyimide Membrane with Fluorine-Containing Diamines for Efficient Gas Separation. Membranes, 12(3), 256. https://doi.org/10.3390/membranes12030256