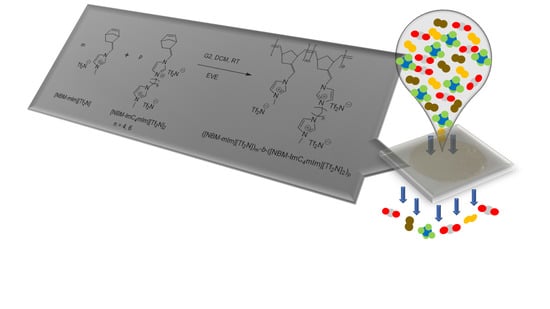
Poly(ionic liquid)s with Dicationic Pendants as Gas Separation Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis
2.3.1. Intermediates 3-(4-Bromo(iodo)alkyl)-1-methyl-1H-imidazol-3-ium Bromide (Iodide) Salts
Synthesis of 3-(4-Bromobutyl)-1-methyl-1H-imidazol-3-ium Bromide [C4BrmIm][Br]
Synthesis of 3-(4-Bromohexyl)-1-methyl-1H-imidazol-3-ium Bromide [C6BrmIm][Br]
Synthesis of 3-(4-Bromodecyl)-1-methyl-1H-imidazol-3-ium Bromide [C10BrmIm][Br]
Synthesis of 3-(2-(2-(2-Iodoethoxy)ethoxy)ethoxy)ethyl)-1-methyl-1H-imidazol-3-ium Iodide [((C2O)3C2I)mIm][I]
2.3.2. Norbornenyl-Containing Imidazolium-Based ILs
Synthesis of Norbornylmethylene Bromide (NBM-Br)
Synthesis of 1-Norbornylmethylene-Imidazole (NBM-Im)
Synthesis of 3-(4-Bromobutyl)-1-norbornylmethylene-1H-imidazol-3-ium Bromide ([NBM-ImC4Br][Br])
Synthesis of 1-Norbornylmethylene-3-methyl-1H-imidazol-3-ium Bistriflimide[NBM-mIm][Tf2N]
Synthesis of 1-Norbornylmethylene-3-(4-(3-methyl-1H-imidazol-3-ium)butyl)-1H-imidazol-3-ium di-bistriflimide [NBM-ImC4mIm][Tf2N]2
Synthesis of 1-Norbornylmethylene-3-(4-(2, 3-dimethyl-1H-imidazol-3-ium)butyl)-1H-imidazol-3-ium di-bistriflimide [NBM-ImC4m(2-m)Im][Tf2N]2
Synthesis of 1-Norbornylmethylene-3-(6-(3-methyl-1H-imidazol-3-ium)hexyl)-1H-imidazol-3-ium di-bistriflimide [NBM-ImC6mIm][Tf2N]2
Synthesis of 1-Norbornylmethylene-3-(10-(3-methyl-1H-imidazol-3-ium)decyl)-1H-imidazol-3-ium di-bistriflimide [NBM-ImC10mIm][Tf2N]2
Synthesis of 1-Norbornylmethylene-3-(2-(2-(2-(2-(3-methyl-1H-imidazol-3-ium)ethoxy)ethoxy)ethoxy)ethyl)-1-methyl-1H-imidazol-3-ium di-bistriflimide [NBM-Im((C2OC2)2O)Im][Tf2N]2
2.4. Polymerization
2.4.1. Homopolymerization
2.4.2. Random Copolymerization
2.4.3. Block Copolymerization
2.5. Gas Seperation Measurements
3. Results and Discussion
3.1. Monomer Characterization
3.2. Polymer Characterization
3.3. Membrane Characterization
3.3.1. Gel Permeation Chromatography (GPC)
3.3.2. Density of Polymers
3.3.3. Wide-Angle X-ray Diffraction (WAXD)
3.3.4. Membrane Casting
4. Gas Separations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew. Sust. Energ. Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Sidhikku Kandath Valappil, R.; Ghasem, N.; Al-Marzouqi, M. Current and future trends in polymer membrane-based gas separation technology: A comprehensive review. J. Ind. Eng. Chem. 2021, 98, 103–129. [Google Scholar] [CrossRef]
- Mahurin, S.M.; Lee, J.S.; Baker, G.A.; Luo, H.; Dai, S. Performance of nitrile-containing anions in task-specific ionic liquids for improved CO2/N2 separation. J. Membr. Sci. 2010, 353, 177–183. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, R.; Ellern, A.; Armstrong, D.W. Structure and properties of high stability geminal dicationic ionic liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Ravula, S.; Larm, N.E.; Mottaleb, M.A.; Heitz, M.P.; Baker, G.A. Vapor pressure mapping of ionic liquids and low-volatility fluids using graded isothermal thermogravimetric analysis. ChemEngineering 2019, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 Capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.E.; Gabriel, C.J.; Lessmann, S.; Carlisle, T.K.; Finotello, A.; Gin, D.L.; Noble, R.D. Enhanced CO2 separation selectivity in oligo (ethylene glycol) functionalized room-temperature ionic liquids. Ind. Eng. Chem. Res. 2007, 46, 5380–5386. [Google Scholar] [CrossRef]
- Camper, D.; Scovazzo, P.; Koval, C.; Noble, R. Gas solubilities in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2004, 43, 3049–3054. [Google Scholar] [CrossRef]
- Gan, Q.; Rooney, D.; Xue, M.; Thompson, G.; Zou, Y. An experimental study of gas transport and separation properties of ionic liquids supported on nanofiltration membranes. J. Membr. Sci. 2006, 280, 948–956. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bonilla, A.; Fernández-García, M. Poly(ionic liquid)s as antimicrobial materials. Eur. Polym. J. 2018, 105, 135–149. [Google Scholar] [CrossRef]
- Zhou, X.; Weber, J.; Yuan, J. Poly (ionic liquid)s: Platform for CO2 capture and catalysis. Curr. Opin. Green Sustain. Chem. 2019, 16, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Elwan, H.A.; Thimmappa, R.; Mamlouk, M.; Scott, K. Applications of poly ionic liquids in proton exchange membrane fuel cells: A review. J. Power Sour. 2021, 510, 230371. [Google Scholar] [CrossRef]
- Friess, K.; Izák, P.; Kárászová, M.; Pasichnyk, M.; Lanč, M.; Nikolaeva, D.; Luis, P.; Jansen, J.C. A Review on Ionic Liquid Gas Separation Membranes. Membranes 2021, 11, 97. [Google Scholar] [CrossRef]
- Zia ul Mustafa, M.; bin Mukhtar, H.; Md Nordin, N.A.H.; Mannan, H.A.; Nasir, R.; Fazil, N. Recent developments and applications of ionic liquids in gas separation membranes. Chem. Eng. Technol. 2019, 42, 2580–2593. [Google Scholar] [CrossRef]
- Rashid, T.U. Ionic liquids: Innovative fluids for sustainable gas separation from industrial waste stream. J. Mol. Liq. 2021, 321, 114916–114941. [Google Scholar] [CrossRef]
- Tang, J.; Sun, W.; Tang, H.; Radosz, M.; Shen, Y. Enhanced CO2 absorption of Poly(ionic liquid)s. Macromolecules 2005, 38, 2037–2039. [Google Scholar] [CrossRef]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and performance of polymerizable room-temperature ionic liquids as gas separation membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Bara, J.E.; Gabriel, C.J.; Hatakeyama, E.S.; Carlisle, T.K.; Lessmann, S.; Noble, R.D.; Gin, D.L. Improving CO2 selectivity in polymerized room-temperature ionic liquid gas separation membranes through incorporation of polar substituents. J. Membr. Sci. 2008, 321, 3–7. [Google Scholar] [CrossRef]
- Bara, J.E.; Hatakeyama, E.S.; Gabriel, C.J.; Zeng, X.; Lessmann, S.; Gin, D.L.; Noble, R.D. Synthesis and light gas separations in cross-linked gemini room temperature ionic liquid polymer membranes. J. Membr. Sci. 2008, 316, 186–191. [Google Scholar] [CrossRef]
- Carlisle, T.K.; Nicodemus, G.D.; Gin, D.L.; Noble, R.D. CO2/light gas separation performance of cross-linked poly(vinylimidazolium) gel membranes as a function of ionic liquid loading and cross-linker content. J. Membr. Sci. 2012, 397–398, 24–37. [Google Scholar] [CrossRef]
- Carlisle, T.K.; Bara, J.E.; Lafrate, A.L.; Gin, D.L.; Noble, R.D. Main-chain imidazolium polymer membranes for CO2 separations: An initial study of a new ionic liquid-inspired platform. J. Membr. Sci. 2010, 359, 37–43. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Kumbharkar, S.C.; Kharul, U.K. Polymeric ionic liquids (PILs): Effect of anion variation on their CO2 sorption. J. Membr. Sci. 2012, 389, 305–315. [Google Scholar] [CrossRef]
- Kammakakam, I.; Bara, J.E.; Jackson, E.M.; Lertxundi, J.; Mecerreyes, D.; Tomé, L.C. Tailored CO2-Philic Anionic Poly(ionic liquid) Composite Membranes: Synthesis, Characterization, and Gas Transport Properties. ACS Sustain. Chem. Eng. 2020, 8, 5954–5965. [Google Scholar] [CrossRef]
- Tomé, L.C.; Aboudzadeh, M.A.; Rebelo, L.P.N.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric ionic liquids with mixtures of counter-anions: A new straightforward strategy for designing pyrrolidinium-based CO2 separation membranes. J. Mater. Chem. A 2013, 1, 10403–10411. [Google Scholar] [CrossRef]
- Bara, J.E.; Gin, D.L.; Noble, R.D. Effect of anion on gas separation performance of polymer−room-temperature ionic liquid composite membranes. Ind. Eng. Chem. Res. 2008, 47, 9919–9924. [Google Scholar] [CrossRef]
- Kammakakam, I.; Bara, J.E.; Jackson, E.M. Dual anion–cation crosslinked poly(ionic liquid) composite membranes for enhanced CO2 separation. ACS Appl. Polym. Mater. 2020, 2, 5067–5076. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymeric gas separation membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Wang, X.; Wilson, T.J.; Alentiev, D.; Gringolts, M.; Finkelshtein, E.; Bermeshev, M.; Long, B.K. Substituted polynorbornene membranes: A modular template for targeted gas separations. Polym. Chem. 2021, 12, 2947–2977. [Google Scholar] [CrossRef]
- García-Loma, R.; Albéniz, A.C. Poly(ω-bromoalkylnorbornenes-co-norbornene) by ROMP-hydrogenation: A robust support amenable to post-polymerization functionalization. RSC Adv. 2015, 5, 70244–70254. [Google Scholar] [CrossRef] [Green Version]
- Gmernicki, K.R.; Hong, E.; Maroon, C.R.; Mahurin, S.M.; Sokolov, A.P.; Saito, T.; Long, B.K. Accessing siloxane functionalized polynorbornenes via vinyl-addition polymerization for CO2 separation membranes. ACS Macro Lett. 2016, 5, 879–883. [Google Scholar] [CrossRef]
- Ye, Q.; Gao, T.; Wan, F.; Yu, B.; Pei, X.; Zhou, F.; Xue, Q. Grafting poly(ionic liquid) brushes for anti-bacterial and anti-biofouling applications. J. Mater. Chem. 2012, 22, 13123–13131. [Google Scholar] [CrossRef]
- Cui, J.; Nie, F.-M.; Yang, J.-X.; Pan, L.; Ma, Z.; Li, Y.-S. Novel imidazolium-based poly(ionic liquid)s with different counterions for self-healing. J. Mater. Chem. A 2017, 5, 25220–25229. [Google Scholar] [CrossRef]
- Wiesenauer, E.F.; Edwards, J.P.; Scalfani, V.F.; Bailey, T.S.; Gin, D.L. Synthesis and ordered phase separation of imidazolium-based alkyl–ionic diblock copolymers made via ROMP. Macromolecules 2011, 44, 5075–5078. [Google Scholar] [CrossRef]
- Biondi, I.; Laurenczy, G.; Dyson, P.J. Synthesis of gold nanoparticle catalysts based on a new water-soluble ionic polymer. Inorg. Chem. 2011, 50, 8038–8045. [Google Scholar] [CrossRef]
- Choi, U.H.; Price, T.L.; Schoonover, D.V.; Xie, R.; Gibson, H.W.; Colby, R.H. Role of chain polarity on ion and polymer dynamics: Molecular volume-based analysis of the dielectric constant for polymerized norbornene-based ionic liquids. Macromolecules 2020, 53, 10561–10573. [Google Scholar] [CrossRef]
- Nie, F.-M.; Cui, J.; Zhou, Y.-F.; Pan, L.; Ma, Z.; Li, Y.-S. Molecular-level tuning toward aggregation dynamics of self-healing materials. Macromolecules 2019, 52, 5289–5297. [Google Scholar] [CrossRef]
- Vygodskii, Y.S.; Shaplov, A.S.; Lozinskaya, E.I.; Lyssenko, K.A.; Golovanov, D.G.; Malyshkina, I.A.; Gavrilova, N.D.; Buchmeiser, M.R. Conductive polymer electrolytes derived from poly(norbornene)s with pendant ionic imidazolium moieties. Macromol. Chem. Phys. 2008, 209, 40–51. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; Zhu, H.; Chen, D. Preparation of a ROMP-type imidazolium-functionalized norbornene ionic liquid block copolymer and the electrochemical property for lithium-ion batteries polyelectrolyte membranes. RSC Adv. 2015, 5, 43581–43588. [Google Scholar] [CrossRef]
- He, X.; Wang, Z.; Zhou, W.; Jiang, X.; Han, Z.; Chen, D. Imidazolium-functionalized norbornene ionic liquid block copolymer and silica composite electrolyte membranes for lithium-ion batteries. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Wiesenauer, E.F.; Gin, D.L.; Noble, R.D. Effect of composition and nanostructure on CO2/N2 transport properties of supported alkyl-imidazolium block copolymer membranes. J. Membr. Sci. 2013, 430, 312–320. [Google Scholar] [CrossRef]
- Wiesenauer, E.F.; Nguyen, P.T.; Newell, B.S.; Bailey, T.S.; Noble, R.D.; Gin, D.L. Imidazolium-containing, hydrophobic–ionic–hydrophilic ABC triblock copolymers: Synthesis, ordered phase-separation, and supported membrane fabrication. Soft Matter 2013, 9, 7923–7927. [Google Scholar] [CrossRef]
- Ouchi, M.; Inoue, Y.; Kanzaki, T.; Hakushi, T. Molecular design of crown ethers. 1. Effects of methylene chain length: 15- to 17-crown-5 and 18- to 22-crown-6. J. Org. Chem. 1984, 49, 1408–1412. [Google Scholar] [CrossRef]
- Li, P.; Paul, D.R.; Chung, T.-S. High performance membranes based on ionic liquid polymers for CO2 separation from the flue gas. Green Chem. 2012, 14, 1052–1063. [Google Scholar] [CrossRef]
- Öztürk, B.Ö.; Durmuş, B.; Karabulut Şehitoğlu, S. Olefin metathesis in air using latent ruthenium catalysts: Imidazole substituted amphiphilic hydrogenated ROMP polymers providing nano-sized reaction spaces in water. Catal. Sci. Technol. 2018, 8, 5807–5815. [Google Scholar] [CrossRef]
- Yu, H.; Lin, S.; Sun, D.; Pan, Q. Synthesis of norbornene derivatives and their polymers via ROMP of norbornene derivatives. High Perform. Polym. 2020, 32, 729–737. [Google Scholar] [CrossRef]
- Sonoda, T.; Kobayashi, S.; Tanaka, M. Periodically Functionalized linear polyethylene with tertiary amino groups via regioselective ring-opening metathesis polymerization. Macromolecules 2021, 54, 2862–2872. [Google Scholar] [CrossRef]
- Swan, S.; Egemole, F.O.; Nguyen, S.T.; Kim, J.-H. Assembly of short-chain amphiphilic homopolymers into well-defined particles. Langmuir 2020, 36, 4548–4555. [Google Scholar] [CrossRef] [PubMed]
- O’Harra, K.E.; Kammakakam, I.; Noll, D.M.; Turflinger, E.M.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Synthesis and performance of aromatic polyamide ionenes as gas separation membranes. Membranes 2020, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]







| ID | Membrane | Feed Ratio a [M]:[DIL] | Density (g/cm3) | WAXD | GPC b | |||
|---|---|---|---|---|---|---|---|---|
| d- Spacing (Å) | FWHM (Deg.) | Mn (Da) | Mw (Da) | PDI (Mn/Mw) | ||||
| HM | p([NBM-mIm][Tf2N) | 1.0:0.0 | 1.24 ± 0.01 | 4.66 | 12.76 ± 0.04 | 32,114 | 64,592 | 2.01 |
| BCP1-C4 | (([NBM-mIm][Tf2N])1-b- ([NBM-ImC4mIm][Tf2N]2)0.11) | 1.0:0.11 | 1.25 ± 0.03 | 4.65 | 10.17 ± 0.05 | 95,822 | 182,939 | 1.91 |
| BCP2-C4 | (([NBM-mIm][Tf2N])1-b- ([NBM-ImC4mIm][Tf2N]2)0.25) | 1.0:0.25 | 1.43 ± 0.07 | 4.75 | 10.74 ± 0.03 | 100,857 | 188,930 | 1.87 |
| BCP1-C6 | (([NBM-mIm][Tf2N])1-b- ([NBM-ImC6mIm][Tf2N]2)0.11) | 1.0:0.11 | 1.47 ± 0.06 | 4.55 | 11.37 ± 0.05 | 62,820 | 132,728 | 2.11 |
| BCP2-C6 | (([NBM-mIm][Tf2N])1-b- ([NBM-ImC6mIm][Tf2N]2)0.25) | 1.0:0.25 | 1.53 ± 0.02 | 4.74 | 10.55 ± 0.03 | 17,389 | 56,603 | 3.25 |
| Membrane | Permeability a,b | Permselectivity b | |||||
|---|---|---|---|---|---|---|---|
| PCO2 | PN2 | PCH4 | PH2 | αCO2/N2 | αCO2/CH4 | αCO2/H2 | |
| HM | 6.34 ± 0.37 | 0.29 ± 0.03 | 0.31 ± 0.02 | 3.98 ± 0.13 | 20.76 ± 0.41 | 20.92 ± 0.37 | 1.59 ± 0.05 |
| BCP1-C4 | 9.61 ± 0.77 | 0.43 ± 0.03 | 0.55 ± 0.01 | 5.54 ± 0.55 | 19.72 ± 0.41 | 17.80 ± 0.76 | 1.69 ± 0.14 |
| BCP2-C4 | 7.26 ± 0.15 | 0.23 ± 0.02 | 0.29 ± 0.04 | 4.92 ± 0.25 | 31.99 ± 0.63 | 27.61± 0.15 | 1.45 ± 0.07 |
| BCP1-C6 | 11.23 ± 0.30 | 0.51 ± 0.01 | 0.58 ± 0.02 | 7.69 ± 0.08 | 21.82 ± 0.97 | 19.61 ± 0.39 | 1.48 ± 0.01 |
| BCP2-C6 | 6.05 ± 0.29 | 0.21 ± 0.05 | 0.34 ± 0.01 | 3.86 ± 0.10 | 30.82 ± 0.37 | 16.28 ± 1.03 | 1.57 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravula, S.; O’Harra, K.E.; Watson, K.A.; Bara, J.E. Poly(ionic liquid)s with Dicationic Pendants as Gas Separation Membranes. Membranes 2022, 12, 264. https://doi.org/10.3390/membranes12030264
Ravula S, O’Harra KE, Watson KA, Bara JE. Poly(ionic liquid)s with Dicationic Pendants as Gas Separation Membranes. Membranes. 2022; 12(3):264. https://doi.org/10.3390/membranes12030264
Chicago/Turabian StyleRavula, Sudhir, Kathryn E. O’Harra, Keith A. Watson, and Jason E. Bara. 2022. "Poly(ionic liquid)s with Dicationic Pendants as Gas Separation Membranes" Membranes 12, no. 3: 264. https://doi.org/10.3390/membranes12030264
APA StyleRavula, S., O’Harra, K. E., Watson, K. A., & Bara, J. E. (2022). Poly(ionic liquid)s with Dicationic Pendants as Gas Separation Membranes. Membranes, 12(3), 264. https://doi.org/10.3390/membranes12030264