All-Solid-State Potentiometric Platforms Modified with a Multi-Walled Carbon Nanotubes for Fluoxetine Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
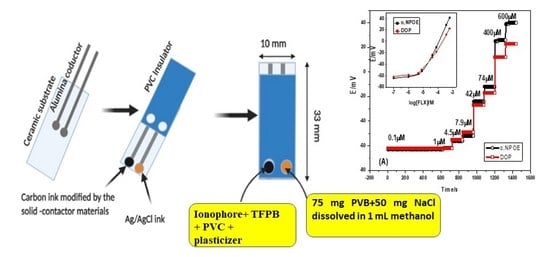
2.3. Electrode Fabrication and Potential Measurements
2.4. Chronopotentiometric Measurements
2.5. Application to Real Samples
3. Results
3.1. Sensors’ Characterizations
3.2. Selectivity Behavior
3.3. Repeatability, Reproducibility, and Stability
3.4. Water-Layer Test
3.5. Short-Term Potential Stability and Interfacial Capacitances
3.6. Analytical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Visentin, A.P.V.; Colombo, R.; Scotton, E.; Fracasso, D.S.; da Rosa, A.R.; Branco, C.S.; Salvador, M. Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxid. Med. Cell. Longev. 2020, 2020, 2972968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severe, J.; Greden, J.F.; Reddy, P. Consequences of Recurrence of Major Depressive Disorder: Is Stopping Effective Antidepressant Medications Ever Safe? Focus 2020, 18, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Latha, M.S.; Sowjanya, B.; Abbulu, K. Analytical method development for the simultaneous estimation of olanzapine and fluoxetine by RP-HPLC method. Int. J. Bio-Pharm. Res. 2019, 8, 2769–2774. [Google Scholar]
- Creeley, C.E.; Denton, L.K. Use of Prescribed Psychotropics during Pregnancy: A Systematic Review of Pregnancy, Neonatal, and Childhood Outcomes. Brain Sci. 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Marazziti, D.; Avella, M.T.; Basile, L.; Mucci, F.; Dell’Osso, L. Pharmacokinetics of serotonergic drugs: Focus on OCD. Expert Opin. Drug Metab. Toxicol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Al-Shalabi, R.; Hefnawy, M.; Al-Johar, H.; Alrabiah, H. Validated UPLC-MS/MS Method for the Simultaneous Quantification of Vortioxetine and Fluoxetine in Plasma: Application to Their Pharmacokinetic Interaction Study in Wistar Rats. AJAC 2020, 11, 233–259. [Google Scholar] [CrossRef]
- Higashi, Y.; Gao, R.; Fujii, Y. Determination of Fluoxetine and Norfluoxetine in Human Serum and Urine by HPLC Using a Cholester Column with Fluorescence Detection. J. Liq. Chromatogr. Relat. 2009, 32, 1141–1151. [Google Scholar] [CrossRef]
- Ghorbani, M.; Esmaelnia, M.; Aghamohammadhasan, M.; Akhlaghi, H.; Seyedin, O.; Azari, A.Z. Preconcentration and Determination Of Fluoxetine and Norfluoxetine in Biological and Water Samples with β-cyclodextrin Multi-walled Carbon Nanotubes as a Suitable Hollow Fiber Solid phase Microextraction Sorbent and High Performance Liquid Chromatography. J. Anal. Chem. 2019, 74, 540–549. [Google Scholar] [CrossRef]
- Wroblewski, K.; Petruczynik, A.; Waksmundzka-Hajnos, M. Separation and determination of selected psychotropic drugs in human serum by SPE/HPLC/DAD on C18 and Polar-RP columns. J. Liq. Chromatogr. Relat. 2017, 40, 75–82. [Google Scholar] [CrossRef]
- Song, L.; Zheng, Z.; Liang, C.; Chen, X.; Zhang, R.; Hong, Z.; Chai, Y. Rapid solid-phase extraction coupled with GC–MS method for the determination of venlafaxine in rat plasma: Application to the drug–drug pharmacokinetic interaction study of venlafaxine combined with fluoxetine. J. Sep. Sci. 2017, 40, 3462–3468. [Google Scholar] [CrossRef]
- Erarpat, S.; Cağlak, A.; Bodur, S.; Chormey, S.D.; Engin, O.G.; Bakırdere, S. Simultaneous Determination of Fluoxetine, Estrone, Pesticides, and Endocrine Disruptors in Wastewater by Gas Chromatography–Mass Spectrometry (GC–MS) Following Switchable Solvent–Liquid Phase Microextraction (SS–LPME). Anal. Lett. 2019, 52, 869–878. [Google Scholar] [CrossRef]
- Urichuk, L.J.; Aspeslet, L.J.; Holt, A.; Silverstone, P.H.; Coutts, R.T.; Baker, G.B. Determination of p-trifluoromethylphenol, a metabolite of fluoxetine, in tissues and body fluids using an electron-capture gas chromatographic procedure. J. Chromatogr. B Biomed. Appl. 1997, 698, 103–109. [Google Scholar] [CrossRef]
- Oliveira, A.F.F.; de Figueiredo, E.C.; dos Santos-Neto, Á.J. Analysis of fluoxetine and norfluoxetine in human plasma by liquid-phase microextraction and injection port derivatization GC–MS. J. Pharm. Biomed. 2013, 73, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bonde, S.; Bhadane, R.; Gaikwad, A.; Gavali, S.R.; Katale, D.U.; Narendiran, A.S. Simultaneous determination of Olanzapine and Fluoxetine in human plasma by LC–MS/MS: Its pharmacokinetic application. J. Pharm. Biomed. 2014, 90, 64–71. [Google Scholar] [CrossRef]
- Ahmad, I.; Ullah, Z.; Khan, M.I.; Alahmari, A.K.; Khan, M.F. Development and validation of an automated solid-phase extraction-LC-MS/MS method for the bioanalysis of fluoxetine in human plasma. J. Adv. Pharm. Technol. 2021, 12, 267–273. [Google Scholar]
- Murtada, K.; de Andrés, F.; Ríos, A.; Zougagh, M. Determination of antidepressants in human urine extracted by magnetic multiwalled carbon nanotube poly(styrene-co-divinylbenzene) composites and separation by capillary electrophoresis. Electrophoresis 2018, 39, 1808–1815. [Google Scholar] [CrossRef]
- Catai, A.P.F.; Carrilho, E.; Lanças, F.M.; Queiroz, M.E.C. Fast separation of selective serotonin reuptake inhibitors antidepressants in plasma sample by nonaqueous capillary electrophoresis. J. Chromatogr. A 2009, 1216, 5779–5782. [Google Scholar] [CrossRef]
- Himmelsbach, M.; Buchberger, W.; Klampfl, C.W. Determination of antidepressants in surface and waste water samples by capillary electrophoresis with electrospray ionization mass spectrometric detection after preconcentration using off-line solid-phase extraction. Electrophoresis 2006, 27, 1220–1226. [Google Scholar] [CrossRef]
- Martín, M.I.G.; Pérez, C.G. Batch and Flow Injection Fluorimetric Determination of Fluoxetine. Anal. Lett. 1997, 30, 2493–2502. [Google Scholar] [CrossRef]
- Darwish, I.A.; Amer, S.M.; Abdine, H.H.; Al-Rayes, L.I. New Spectrophotometric and Fluorimetric Methods for Determination of Fluoxetine in Pharmaceutical Formulations. Int. J. Anal. Chem. 2009, 2009, 257306. [Google Scholar] [CrossRef] [Green Version]
- Bigdelifam, D.; Mirzaei, M.; Hashemi, M.; Amoli-Diva, M.; Rahmani, O.; Zohrabi, P.; Taherimaslak, Z.; Turkjokar, M. Sensitive spectrophotometric determination of fluoxetine from urine samples using charge transfer complex formation after solid phase extraction by magnetic multiwalled carbon nanotubes. Anal. Methods 2014, 6, 8633–8639. [Google Scholar] [CrossRef]
- Nezhadali, A.; Motlagh, M.O.; Sadeghzadeh, S. Spectrophotometric determination of fluoxetine by molecularly imprinted polypyrrole and optimization by experimental design, artificial neural network and genetic algorithm. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 190, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.K.; Patel, J.N.; Jani, G.K.; Prajapati, L.M.; Gagoria, J. First derivative spectrophotometric determination of fluoxetine hydrochloride and olanzapine in tablets. Int. J. Pharm. Sci. Res. 2011, 2, 2996–3001. [Google Scholar]
- Lencastre, R.P.; Delerue-Matos, C.; Garrido, J.; Borges, F.; Garrido, E.M. Voltammetric quantification of fluoxetine: Application to quality control and quality assurance processes. J. Food Drug Anal. 2006, 14, 242–246. [Google Scholar] [CrossRef]
- Da Silva, A.M.S.R.; Lima, J.C.; Teles, M.T.O.; Brett, A.M.O. Electrochemical studies and square wave adsorptive stripping voltammetry of the antidepressant fluoxetine. Talanta 1999, 49, 611–617. [Google Scholar] [CrossRef]
- Nouws, H.P.; Delerue-Matos, C.; Barros, A.A.; Rodrigues, J.A.; Santos-Silva, A.; Borges, F. Square-Wave Adsorptive-Stripping Voltammetric Detection in the Quality Control of Fluoxetine. Anal. Lett. 2007, 40, 1131–1146. [Google Scholar] [CrossRef]
- Alizadeh, T.; Azizi, S. Graphene/graphite paste electrode incorporated with molecularly imprinted polymer nanoparticles as a novel sensor for differential pulse voltammetry determination of fluoxetine. Biosens. Bioelectron. 2016, 81, 198–206. [Google Scholar] [CrossRef]
- Mostafa, G.A.E.; Hefnawy, M.M.; El-Majed, A. PVC Membrane Sensor for Potentiometric Determination of Fluoxetine in Some Pharmaceutical Formulations. Instrum. Sci. Technol. 2008, 36, 279–290. [Google Scholar] [CrossRef]
- Hussien, E.; Abdel-Gawad, F.; Issa, Y. Ion-selective electrodes for determination of fluoxetine in capsules and in biological fluids. Biochem. Eng. J. 2011, 53, 210–215. [Google Scholar] [CrossRef]
- Arvand, M.; Rad, N.A. Determination of fluoxetine in pharmaceutical preparations and biological samples using potentiometric sensors based on polymeric membranes. J. Anal. Chem. 2013, 68, 183–188. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Amr, A.E.-G.E.; Hashem, H.M.; Abdel Bary, E.M. Imprinted Polymeric Beads-Based Screen-Printed Potentiometric Plat forms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) for Selective Recognition of Fluoxetine. Nanomaterials 2020, 10, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, A.H.; Sayour, H.E.M. Flow-through assay of quinine using solid contact potentiometric sensors based on molecularly imprinted polymers. Electroanalysis 2009, 21, 2701–2708. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Kamel, A.H.; Sales, M.G.F. FIA potentiometric system based on periodate polymeric membrane sensors for the assessment of ascorbic acid in commercial drinks. Food Chem. 2010, 120, 934–939. [Google Scholar] [CrossRef] [Green Version]
- Kamel, A.H.; Galal, H.R. MIP-based biomimetic sensors for static and hydrodynamic potentiometric transduction of sitagliptin in biological fluids. Int. J. Electrochem. Sci. 2014, 9, 4361–4373. [Google Scholar]
- Kamel, A.H.; Galal, H.R.; Hanna, A.A. Novel Planar Chip Biosensors for Potentiometric Immunoassay of Acid Phosphatase Activity Based on the Use of Ion Association Complexes as Novel Electroactive Materials. Int. J. Electrochem. Sci. 2014, 9, 5776–5787. [Google Scholar]
- Abdalla, N.S.; Amr, A.E.-G.E.; El-Tantawy, A.S.M.; Al-Omar, M.O.; Kamel, A.H.; Khalifa, N.M. Tailor-made specific recognition of cyromazine pesticide integrated in a potentiometric strip cell for environmental and food analysis. Polymers 2019, 11, 1526. [Google Scholar] [CrossRef] [Green Version]
- Ezzat, S.; Ahmed, M.A.; Amr, A.E.-G.E.; Al-Omar, M.O.; Kamel, A.H.; Khalifa, N.M. Single-Piece All-Solid-State Potential Ion-Selective Electrodes Integrated with Molecularly Imprinted Polymers (MIPs) for Neutral 2, 4-Dichlorophenol Assessment. Materials 2019, 12, 2924. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.M.; Mahmoud, W.H.; Mohamed, A.H.K.; Kelany, A.E. Mercury(II) ion-selective polymeric membrane sensors for analysis of mercury in hazardous wastes. Anal. Sci. 2006, 22, 877–881. [Google Scholar] [CrossRef] [Green Version]
- Galal Eldin, A.; Amr, A.E.-G.E.; Kamel, A.H.; Hassan, S.S.M. Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer as Solid Transducer for Ultratrace Determination of Azides. Molecules 2019, 24, 1392. [Google Scholar] [CrossRef] [Green Version]
- Buck, R.P.; Lindner, E. IUPAC recommendations for nomenclature of ion-selective electrodes. Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Lindner, E.; Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro-and micro fabricated ion-selective sensors. Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Botella, S.; Del Castillo, B.; Martin, M. Cyclodetrin properties and applications of inclusion complex formation. Ars Pharm 1995, 36, 187–198. [Google Scholar]
- Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Abu-Shawish, H.M. A mercury (II) selective sensor based on N, N′-bis (salicylaldehyde)-phenylenediamine as neutral carrier for potentiometric analysis in water samples. J. Hazard. Mat. 2009, 167, 602–608. [Google Scholar] [CrossRef]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric Selectivity Coefficients of Ion-Selective Electrodes. Part I. Inorganic Cations (Technical Report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Takeda, Y.; Kato, H. The solvent extraction of bivalent metal picrates by 15-crown-5, 18-crown-6, and dibenzo-18-crown-6. Chem. Soc. Jap. 1979, 52, 1027–1030. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, S.; Zhou, Y.; Guan, S.; Zhang, L. Inclusion complexes of fluconazole with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin in aqueous solution: Preparation, characterization and a structural insight. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 209–217. [Google Scholar] [CrossRef]
- Abu-Shawish, H.M.; Elhabiby, M.; Abu Aziz, H.S.; Saadeh, S.M.; Tbaza, A. Determination of Trihexyphenidyl hydrochloride drug in tablets and urine using a potentiometric carbon paste electrode. Sens. Actuators B 2016, 235, 18–26. [Google Scholar] [CrossRef]
- Veder, J.P.; De Marco, R.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Elimination of undesirable water layers in solid-contact polymeric ion-selective electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef] [Green Version]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- British Pharmacopoeia, (Ph. Eur. Monograph 0522), Medicines and Healthcare Products Regulatory Agency (MHRA). 2013. Available online: https://www.pharmacopoeia.com (accessed on 2 April 2021).







| Sensing Material | Electrode Type | Slope, mV/DECADE | Detection Limit, M | Lower Limit of Linear Range, M | Working pH Range | Ref. |
|---|---|---|---|---|---|---|
| Fluoxetine/picrolonate | Liquid polymeric | 51 ± 0.5 | 6 × 10−6 | 8 × 10−6 | 1–5 | 28 |
| Fluoxetine/tetraphenylborate | Liquid polymeric | 58.5 | 2.3 × 10−5 | 4.3 × 10−5 | 4.0–7.5 | 29 |
| Coated wire graphite electrode | 55.5 | 2.5 × 10−5 | 5.4 × 10−5 | |||
| Fluoxetine/tetraphenylborate | Liquid polymeric | 51 | 3.0 × 10−6 | 3.0 × 10−6 | 4.0–7.5 | 30 |
| Fluoxetine/phosphotungstate | 51.8 | 5.0 × 10−6 | 5.0 × 10−6 | |||
| Molecular imprinting polymer (MIP), acrylamide | Solid-contact ISEs | 58.9 ± 0.2 | 2.1 × 10−6 | 1.0 × 10−5 | 10 mM acetate buffer of pH 4.5 | 31 |
| Ionophore I | Solid-contact ISEs | 56.2 ± 0.8 | 5.2 × 10−6 | 6.5 × 10−6 | 10 mM Tris buffer solution of pH 7 | This work |
| Ionophore II | 56.3 ± 1.7 | 4.7 × 10−6 | 5.6 × 10−6 | |||
| Ionophore III | 64.4 ± 0.2 | 2.0 × 10−7 | 2.0 × 10−7 |
| Parameters | Ionophore I | Ionophore II | Ionophore III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| o-NPOE | DOP | DBS | o-NPOE | DOP | DBS | o-NPOE | DOP | DBS | |
| Slope (mV/decade) | 56.2 ± 0.8 | 40.5 ± 1.2 | 40.4 ± 0.7 | 56.3 ± 1.7 | 52.2 ± 1.4 | 53.6 ± 0.3 | 64.4 ± 0.2 | 48.4 ± 0.7 | 43 ± 0.4 |
| Detection limit (M) | 5.2 × 10−6 | 6.0 × 10−6 | 1.0 × 10−5 | 4.7 × 10−6 | 6.3 × 10−6 | 2.0 × 10−5 | 2.0 × 10−7 | 8.0 × 10−7 | 3.2 × 10−6 |
| Correlation coefficient (R2) | 0.999 | 0.999 | 0.997 | 0.999 | 0.999 | 0.998 | 0.999 | 0.997 | 0.998 |
| Linear range (M) | 6.5 × 10−6–1.0 × 10−2 | 6.5 × 10−6–1.0 × 10−2 | 4.0 × 10−5–1.0 × 10−2 | 5.6 × 10−6–1.0 × 10−2 | 4.5 × 10−6–1.0 × 10−2 | 4.6 × 10−5–1.0 × 10−2 | 6.0 × 10−7–1.0 × 10−2 | 3.2 × 10−6–1.0 × 10−2 | 1.0 × 10−5–1.0 × 10−2 |
| pH range (pH) | 4.5–8.5 | 4.5–8.5 | 4.5–8.5 | 4–9 | 4–9 | 4–9 | 4–9 | 4–9 | 4–9 |
| Precision (mV %) | 1.2 | 1.4 | 1.5 | 1.3 | 1.5 | 1.2 | 1.3 | 0.9 | 1.1 |
| Accuracy (mV %) | 99.8 | 99.0 | 99.1 | 99.7 | 99.8 | 99.1 | 98.8 | 99.2 | 98.5 |
| Standard deviation (mV) | 0.5 | 0.4 | 0.6 | 0.5 | 0.8 | 0.9 | 0.4 | 0.3 | 0.7 |
| Interfering Ion, J | Log KPot FLX, J + SD * | ||
|---|---|---|---|
| Ionophore I | Ionophore II | Ionophore III | |
| Li+ | −5.1 ± 0.1 | −4.5 ± 0.1 | −4.8 ± 0.3 |
| Na+ | −3.7 ± 0.1 | −3.6 ± 0.2 | −4.0 ± 0.2 |
| K+ | −2.9 ± 0.3 | −2.3 ± 0.2 | −3.1 ± 0.1 |
| Rb+ | −4.0 ± 0.1 | −2.5 ± 0.1 | −3.5 ± 0.4 |
| Ca2+ | −5.1 ± 0.1 | −4.6 ± 0.1 | −4.5 ± 0.2 |
| Zn2+ | −4.7 ± 0.2 | −4.4 ± 0.3 | −4.7 ± 0.1 |
| Ba2+ | −5.3 ± 0.2 | −4.9 ± 0.2 | −4.6 ± 0.2 |
| Arginine | −5.8 ± 0.4 | −4.3 ± 0.2 | −5.6 ± 0.3 |
| Caffeine | −5.2 ± 0.1 | −4.2 ± 0.1 | −5.1 ± 0.1 |
| Glucose | −5.0 ± 0.1 | −4.4 ± 0.2 | −3.9 ± 0.3 |
| Lactose | −4.6 ± 0.2 | −3.7 ± 0.1 | −3.3 ± 0.2 |
| Paracetamol | −5.4 ± 0.3 | −3.9 ± 0.2 | −4.9 ± 0.2 |
| Norfluoxetine | −1.0 ± 0.6 | −0.7 ± 0.02 | −1.5 ± 0.1 |
| Ionophore I | Ionophore II | Ionophore III | ||||
|---|---|---|---|---|---|---|
| Without MWCNTs | With MWCNTs | Without MWCNTs | With MWCNTs | Without MWCNTs | With MWCNTs | |
| Potential drift (ΔE/Δt), µV/s | 815.3 ± 3.4 | 95.9 ± 1.1 | 88.9 ± 1.5 | 19.4 ± 1.1 | 120.5 ± 2.1 | 24.6 ± 1.4 |
| CL, µF | 1.2 ± 0.7 | 10.4 ± 0.2 | 11.2 ± 2.6 | 51.5 ± 2.6 | 8.29 ± 1.3 | 40.6 ± 2.1 |
| Pharmaceutical Product and Source | Nominal Content Taken, mg/Tablet | Found, mg/Tablet | t-Student Test b | F-Test | |||
|---|---|---|---|---|---|---|---|
| Proposed Method | Mean a (%) ± SD | Reference Method, [47] | Mean a (%) ± SD | ||||
| Prozac (Lilly, France) | 20 | 20.04 | 100.2 ± 0.4 | 20.1 | 100.8 ± 0.6 | 1.62 | 2.24 |
| Philozac (Amoun, Egypt) | 20 | 19.93 | 99.7 ± 0.6 | 19.8 | 99.07 ± 1.7 | 0.38 | 9.35 |
| Flutin (Eipico, Egypt) | 20 | 20.21 | 101.0 ± 1.4 | 19.8 | 99.4 ± 0.9 | 3.69 | 2.66 |
| Depreban (Amirya, Egypt) | 20 | 19.72 | 98.6 ± 0.8 | 19.4 | 97.2 ± 0.8 | 2.13 | 1.08 |
| Pharmaceutical Product and Source | Nominal Content Taken, mg/Tablet | Found, mg/Tablet | t-Student Test b | F-Test | |||
|---|---|---|---|---|---|---|---|
| Proposed Method | Mean a (%) ± SD | Reference Method | Mean a (%) ± SD | ||||
| Prozac (Lilly, France) | 20 | 20.9 | 102.2 ± 1.4 | 20.1 | 100.8 ± 0.6 | 2.62 | 3.24 |
| Philozac (Amoun, Egypt) | 20 | 18.9 | 99.2 ± 0.8 | 19.8 | 99.07 ± 1.7 | 1.38 | 6.87 |
| Flutin (Eipico, Egypt) | 20 | 18.8 | 102.0 ± 1.8 | 19.8 | 99.4 ± 0.9 | 2.89 | 4.54 |
| Depreban (Amirya, Egypt) | 20 | 20.7 | 102.6 ± 1.5 | 19.4 | 97.2 ± 0.8 | 1.45 | 2.07 |
| Pharmaceutical Product and Source | Nominal Content Taken, mg/Tablet | Found, mg/Tablet | t-Student Test b | F-Test | |||
|---|---|---|---|---|---|---|---|
| Proposed Method | Mean a (%) ± SD | Reference Method | Mean a (%) ± SD | ||||
| Prozac (Lilly, France) | 20 | 19.7 | 98.5 ± 0.4 | 20.1 | 100.8 ± 0.6 | 2.85 | 3.12 |
| Philozac (Amoun, Egypt) | 20 | 20.9 | 104.5 ± 0.5 | 19.8 | 99.07 ± 1.7 | 3.138 | 5.24 |
| Flutin (Eipico, Egypt) | 20 | 19.3 | 96.5 ± 0.8 | 19.8 | 99.4 ± 0.9 | 2.93 | 3.37 |
| Depreban (Amirya, Egypt) | 20 | 19.5 | 97.5 ± 0.4 | 19.4 | 97.2 ± 0.8 | 2.34 | 2.16 |
| Sample No. | Amount of FLX Added, μM | Ionophore I | Ionophore II | Ionophore III | |||
|---|---|---|---|---|---|---|---|
| Amount of FLX Found, μM a | Recovery, % | Amount of FLX Found, μM a | Recovery, % | Amount of FLX Found, μM a | Recovery, % | ||
| 1 | 8.0 | 7.8 ± 0.8 | 97.5 | 7.7 ± 0.9 | 96.3 | 7.8 ± 0.8 | 97.5 |
| 2 | 10.0 | 9.7 ± 0.6 | 97.0 | 9.5 ± 0.4 | 95.0 | 9.8 ± 0.4 | 98.0 |
| 3 | 15.0 | 15.5 ± 0.2 | 103.3 | 15.1 ± 0.3 | 100.6 | 14.8 ± 0.3 | 98.6 |
| 4 | 20.0 | 19.6 ± 0.7 | 98.0 | 20.1 ± 0.6 | 100.5 | 19.8 ± 0.1 | 99.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Rabboh, H.S.M.; M. Hashem, H.; M. S. Al Shagri, L.; E. Amr, A.E.-G.; Almehizia, A.A.; Naglah, A.M.; H. Kamel, A. All-Solid-State Potentiometric Platforms Modified with a Multi-Walled Carbon Nanotubes for Fluoxetine Determination. Membranes 2022, 12, 446. https://doi.org/10.3390/membranes12050446
Abd-Rabboh HSM, M. Hashem H, M. S. Al Shagri L, E. Amr AE-G, Almehizia AA, Naglah AM, H. Kamel A. All-Solid-State Potentiometric Platforms Modified with a Multi-Walled Carbon Nanotubes for Fluoxetine Determination. Membranes. 2022; 12(5):446. https://doi.org/10.3390/membranes12050446
Chicago/Turabian StyleAbd-Rabboh, Hisham S. M., Heba M. Hashem, Layla M. S. Al Shagri, Abdel El-Galil E. Amr, Abdulrahman A. Almehizia, Ahmed M. Naglah, and Ayman H. Kamel. 2022. "All-Solid-State Potentiometric Platforms Modified with a Multi-Walled Carbon Nanotubes for Fluoxetine Determination" Membranes 12, no. 5: 446. https://doi.org/10.3390/membranes12050446
APA StyleAbd-Rabboh, H. S. M., M. Hashem, H., M. S. Al Shagri, L., E. Amr, A. E. -G., Almehizia, A. A., Naglah, A. M., & H. Kamel, A. (2022). All-Solid-State Potentiometric Platforms Modified with a Multi-Walled Carbon Nanotubes for Fluoxetine Determination. Membranes, 12(5), 446. https://doi.org/10.3390/membranes12050446