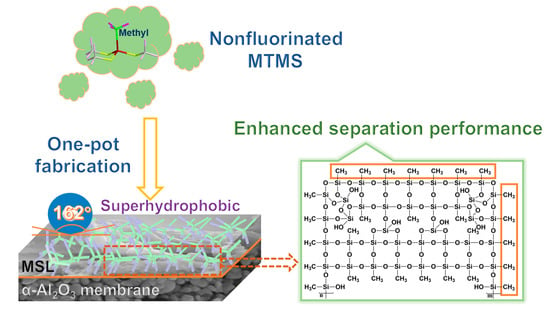
A Methyl-Modified Silica Layer Supported on Porous Ceramic Membranes for the Enhanced Separation of Methyl Tert-Butyl Ether from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of MSL-Supported α-Al2O3 Ceramic Membranes
2.3. Material Characterization
2.4. Vacuum Pervaporation
3. Results and Discussion
3.1. Evolution of MTMS Precursor and Formation of MSL during the Sol-Gel Reaction
3.2. Characterization of MSL-Supported α-Al2O3 Ceramic Membranes
3.3. Separation of MTBE from Aqueous Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achiou, B.; Beqqour, D.; Elomari, H.; Bouazizi, A.; Ouammou, M.; Bouhria, M.; Aaddane, A.; Khiat, K.; Younssi, S.A. Preparation of inexpensive NaA zeolite membrane on pozzolan support at low temperature for dehydration of alcohol solutions. J. Environ. Chem. Eng. 2018, 6, 4429–4437. [Google Scholar] [CrossRef]
- Perego, C.; Bagatin, R.; Tagliabue, M.; Vignola, R. Zeolites and related mesoporous materials for multi-talented environmental solutions. Microporous Mesoporous Mater. 2013, 166, 37–49. [Google Scholar] [CrossRef]
- Yoshida, W.; Cohen, Y. Removal of methyl tert-butyl ether from water by pervaporation using ceramic-supported polymer membranes. J. Membr. Sci. 2004, 229, 27–32. [Google Scholar] [CrossRef]
- He, Z.; Li, G.; Chen, J.; Huang, Y.; An, T.; Zhang, C. Pollution characteristics and health risk assessment of volatile organic compounds emitted from different plastic solid waste recycling workshops. Environ. Int. 2015, 77, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Groves, G.W. Lange’s Chemistry Handbook, 15th ed.; McGraw-Hill Professional: New York, NY, USA, 1998. [Google Scholar]
- Rutkiewicz, I.; Kujawski, W.; Namieśnik, J. Pervaporation of volatile organohalogen compounds through polydimethylsiloxane membrane. Desalination 2010, 64, 160–164. [Google Scholar] [CrossRef]
- Tong, R.; Zhang, L.; Yang, X.; Liu, J.; Zhou, P.; Li, J. Emission characteristics and probabilistic health risk of volatile organic compounds from solvents in wooden furniture manufacturing. J. Clean. Prod. 2019, 208, 1096–1108. [Google Scholar] [CrossRef]
- Kujawski, W.; Krajewska, S.; Kujawski, M.; Gazagnes, L.; Larbot, A.; Persin, M. Pervaporation properties of fluoroalkylsilane (FAS) grafted ceramic membranes. Desalination 2007, 205, 75–86. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, G.; Liu, S.; Liu, Z.; Jin, W. High performance ceramic hollow fiber supported PDMS composite pervaporation membrane for bio-butanol recovery. J. Membr. Sci. 2014, 450, 38–47. [Google Scholar] [CrossRef]
- Kujawa, J.; Al-Gharabli, S.; Kujawski, W.; Knozowska, K. Molecular Grafting of Fluorinated and Nonfluorinated Alkylsiloxanes on Various Ceramic Membrane Surfaces for the Removal of Volatile Organic Compounds Applying Vacuum Membrane Distillation. ACS Appl. Mater. Inter. 2017, 9, 6571–6590. [Google Scholar] [CrossRef] [PubMed]
- He, K.-Y.; Wei, Q.; Wang, Y.-L.; Wang, S.; Cui, S.-P.; Li, Q.-Y.; Nie, Z.-R. Hydrophobic mesoporous organosilica membranes: Preparation and application in the separation of volatile organic compounds from water. Microporous Mesoporous Mater. 2019, 288, 109606. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Removal of hazardous volatile organic compounds from water by vacuum pervaporation with hydrophobic ceramic membranes. J. Membr. Sci. 2015, 474, 11–19. [Google Scholar] [CrossRef]
- Kujawski, W.; Kujawa, J.; Wierzbowska, E.; Cerneaux, S.; Bryjak, M.; Kujawski, J. Influence of hydrophobization conditions and ceramic membranes pore size on their properties in vacuum membrane distillation of water–organic solvent mixtures. J. Membr. Sci. 2016, 499, 442–451. [Google Scholar] [CrossRef]
- Pinnau, I.; Freeman, B.D. Formation and Modification of Polymeric Membranes: Overview, Membrane Formation and Modification; American Chemical Society (ACS): Washington, DC, USA, 1999; pp. 1–22. [Google Scholar]
- Zheng, P.-Y.; Li, X.-Q.; Wu, J.-K.; Wang, N.-X.; Li, J.; An, Q.-F. Enhanced butanol selectivity of pervaporation membrane with fluorinated monolayer on polydimethylsiloxane surface. J. Membr. Sci. 2018, 548, 215–222. [Google Scholar] [CrossRef]
- Borisov, I.; Podtynnikov, I.; Grushevenko, E.; Scharova, O.; Anokhina, T.; Makaev, S.; Volkov, A.; Volkov, V. High Selective Composite Polyalkylmethylsiloxane Membranes for Pervaporative Removal of MTBE from Water: Effect of Polymer Side-chain. Polymers 2020, 12, 1213. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Q.G.; Han, G.L.; Zhu, A.M.; Liu, Q.L. Pervaporation of water–ethanol and methanol–MTBE mixtures using poly (vinyl alcohol)/cellulose acetate blended membranes. J. Membr. Sci. 2013, 448, 93–101. [Google Scholar] [CrossRef]
- Araki, S.; Gondo, D.; Imasaka, S.; Yamamoto, H. Permeation properties of organic compounds from aqueous solutions through hydrophobic silica membranes with different functional groups by pervaporation. J. Membr. Sci. 2016, 514, 458–466. [Google Scholar] [CrossRef]
- Kujawa, J.; Kujawski, W.; Cyganiuk, A.; Dumée, L.F.; Al-Gharabli, S. Upgrading of zirconia membrane performance in removal of hazardous VOCs from water by surface functionalization. Chem. Eng. J. 2019, 374, 155–169. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Highly hydrophobic ceramic membranes applied to the removal of volatile organic compounds in pervaporation. Chem. Eng. J. 2015, 260, 43–54. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Shieh, J.J. Crosslinked blended poly(vinyl alcohol) N-methylol nylon-6 membranes for the pervaporation separation of ethanol-water mixtures. J. Appl. Poly. Sci. 1998, 70, 317–327. [Google Scholar] [CrossRef]
- Xu, P.; Li, X. Fabrication of TiO2/SiO2 superhydrophobic coating for efficient oil/water separation. J. Environ. Chem. Eng. 2021, 9, 105538. [Google Scholar] [CrossRef]
- Sawamura, K.-I.; Furuhata, T.; Sekine, Y.; Kikuchi, E.; Subramanian, B.; Matsukata, M. Zeolite Membrane for Dehydration of Isopropylalcohol-Water Mixture by Vapor Permeation. ACS Appl. Mater. Inter. 2015, 7, 13728–13730. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sun, K.; Zhang, G.; Yang, F.; Lin, S.; Dong, Y. Robust zirconia ceramic membrane with exceptional performance for purifying nano-emulsion oily wastewater. Water Res. 2022, 208, 117859. [Google Scholar] [CrossRef]
- Khalid, A.; Ibrahim, A.; Al-Hamouz, O.C.S.; Laoui, T.; Benamor, A.; Atieh, M.A. Fabrication of polysulfone nanocomposite membranes with silver-doped carbon nanotubes and their antifouling performance. J. Appl. Polym. Sci. 2017, 134, 44468. [Google Scholar] [CrossRef]
- Fang, J.; Qin, G.; Wei, W.; Zhao, X.; Jiang, L. Elaboration of new ceramic membrane from spherical fly ash for microfiltration of rigid particle suspension and oil-in-water emulsion. Desalination 2013, 311, 113–126. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, T.; Xia, Q.; Yu, X.; Wang, Y. Constructing superhydrophobic ZIF-8 layer with bud-like surface morphology on PDMS composite membrane for highly efficient ethanol/water separation. J. Environ. Chem. Eng. 2021, 9, 104977. [Google Scholar] [CrossRef]
- Xu, X.; Nikolaeva, D.; Hartanto, Y.; Luis, P. MOF-based membranes for pervaporation. Sep. Purif. Technol. 2021, 278, 191233. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shimidzu, T.; Maeda, Y.; Magara, K.; Tsugaya, H. Pervaporation of aqueous acetic-acid mixture through poly (acrylic acid-co-acrylonitrile) membranes. J. Membr. Sci. 1993, 83, 157–162. [Google Scholar] [CrossRef]
- Koonaphapdeelert, S.; Li, K. Preparation and characterization of hydrophobic ceramic hollow fibre membrane. J. Membr. Sci. 2007, 291, 70–76. [Google Scholar] [CrossRef]
- Kujawa, J.; Rozicka, A.; Cerneaux, S.; Kujawski, W. The influence of surface modification on the physicochemical properties of ceramic membranes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 567–575. [Google Scholar] [CrossRef]
- Salehi, E.; Madaeni, S.S.; Shamsabadi, A.A.; Laki, S. Applicability of ceramic membrane filters in pretreatment of coke-contaminated petrochemical wastewater: Economic feasibility study. Ceram. Int. 2014, 40, 4805–4810. [Google Scholar] [CrossRef]
- Hosseinabadi, S.R.; Wyns, K.; Buekenhoudt, A.; Van der Bruggen, B.; Ormerod, D. Performance of Grignard functionalized ceramic nanofiltration membranes. Sep. Purif. Technol. 2015, 147, 320–328. [Google Scholar] [CrossRef]
- Jha, S.K.; Mishra, V.K.; Sharma, D.K.; Damodaran, T. Toxicology, Fluoride in the environment and its metabolism in humans. Environ. Contam. Trans. 2011, 211, 121–142. [Google Scholar]
- Wang, Z.; Hao, L.; Yang, F.; Wei, Q. Mesoporous Silica Membranes Silylated by Fluorinated and Non-Fluorinated Alkylsilanes for the Separation of Methyl Tert-Butyl Ether from Water. Membranes 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, L.; Xin, Z. Preparation and Surface Properties of Novel Low Surface Free Energy Fluorinated Silane-Functional Polybenzoxazine Films. Langmuir 2011, 27, 8365–8370. [Google Scholar] [CrossRef]
- Kickelbick, G. Hybrid inorganic-organic mesoporous materials. Angew. Chem. Int. Ed. Engl. 2004, 43, 3102–3104. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, Y.; Ma, K.; Wei, Q.; Nie, Z. A mesoporous SiO2/dense SiO2/Fe3O4 multiply coated hollow microsphere: Synthesis and application on papain immobilization. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.-Y.; Sun, H.; Sun, S.; Liu, J.-G.; Cui, S.-P.; Nie, Z.-R. Synthesis and photocatalytic performance of a novel hollow network Fe3O4/SiO2/meso-TiO2 (FSmT) composite microspheres. J. Sol-Gel Sci. Technol. 2019, 90, 339–347. [Google Scholar] [CrossRef]
- EL Alouani, M.; Alehyen, S.; EL Achouri, M.; Hajjaji, A.; Ennawaoui, C. Influence of the Nature and Rate of Alkaline Activator on the Physicochemical Properties of Fly Ash-Based Geopolymers. Adv. Civ. Eng. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Dur, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Res. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Yang, D.; Li, J.; Xu, Y.; Wu, D.; Sun, Y.; Zhu HDeng, F. Direct formation of hydrophobic silica-based micro/mesoporous hybrids from polymethylhydrosiloxane and tetraethoxysilane. Microporous Mesoporous Mater. 2006, 95, 180–186. [Google Scholar] [CrossRef]
- Wei, Q.; Ding, Y.L.; Nie, Z.R.; Liu, X.G.; Li, Q.Y. Wettability, pore structure and performance of perfluorodecyl-modified silica membranes. J. Membr. Sci. 2014, 466, 114–122. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, Y.; Liu, Y.; Vequizo, J.J.M.; Sun, H.; Yang, C.; Yamakata, A.; Fan, F.; Lin, W.; Wang, X.; et al. Heteroatom Dopants Promote Two-Electron O2 Reduction for Photocatalytic Production of H2 O2 on Polymeric Carbon Nitride. Angew Chem. Int. Ed. Eng. 2020, 59, 16209–16217. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, R.; Jawaharsingh, C.B.; Avila, S.; Ganapathy, K.; Eliel, E.L.; Manoharan, M.; Morris-Natschke SKenan Jr, W.R. Conformational and conFigureurational studies on 3-azabicyclo [3.3.1] nonane (3-abn) derivatives and related systems employing carbon-13 NMR spectroscopy. J. Heterocyclic Chem. 1982, 19, 449–458. [Google Scholar] [CrossRef]
- Sing, K.S.W. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76, 3–11. [Google Scholar] [CrossRef]
- Pinnau, I.; Wijmans, J.G.; Blume, I.; Kuroda, T.; Peinemann, K.V. Gas permeation through composite membranes. J. Membr. Sci. 1988, 37, 81–88. [Google Scholar] [CrossRef]
- Nakae, H.; Inui, R.; Hirata, Y.; Saito, H. Effects of surface roughness on wettability. Acta Mater. 1998, 46, 2313–2318. [Google Scholar] [CrossRef]
- Kakade, B.A.; Pillai, V.K. Tuning the wetting properties of multiwalled carbon nanotubes by surface functionalization. J. Phys. Chem. C 2008, 112, 3183–3186. [Google Scholar] [CrossRef]
- Baghdadi, M. UT (University of Tehran) isotherm as a novel and useful adsorption isotherm for investigation of adsorptive removal of pollutants. J. Environ. Chem. Eng. 2017, 5, 1906–1919. [Google Scholar] [CrossRef]
- Rácz, G.; Kerker, S.; Kovács, Z.; Vatai, G.; Ebrahimi, M.; Czermak, P. Theoretical and experimental approaches of liquid entry pressure determination in membrane distillation processes. Period. Polytech. Chem. Eng. 2014, 58, 81–91. [Google Scholar] [CrossRef] [Green Version]
- da Silva, D.A.R.O.; Zuge, L.C.B.; de Paula Scheer, A. Pretreatments for seawater desalination by pervaporation using the developed green silica/PVA membrane. J. Environ. Chem. Eng. 2021, 9, 106327. [Google Scholar] [CrossRef]
- Putz, A.-M.; Almásy, L.; Len, A.; Ianăşi, C. Functionalized silica materials synthesized via co-condensation and post-grafting methods. Fuller. Nanotub. Carbon Nanostructures 2019, 27, 323–332. [Google Scholar] [CrossRef]
- Feher, F.J.; Newman, D.A. Enhanced silylation reactivity of a model for silica surfaces. J. Am. Chem. Soc. 1990, 112, 1931–1936. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Tsuru, T. Preparation of organic–inorganic hybrid silica membranes using organoalkoxysilanes: The effect of pendant groups. J. Membr. Sci. 2011, 379, 287–295. [Google Scholar] [CrossRef]
- Campaniello, J.; Engelen, C.W.; Haije, W.G.; Pex, P.P.; Vente, J.F. Long-term pervaporation performance of microporous methylated silica membranes. Chem. Commun. 2004, 7, 834–835. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, J.; Rozicka, A.; Bryjak, M.; Kujawski, W. Pervaporative removal of acetone, butanol and ethanol from binary and multicomponent aqueous mixtures. Sep. Puri. Technol. 2014, 132, 422–429. [Google Scholar] [CrossRef]







| Samples | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| Silica microspheres | 736.0 | 1.3 | 8.8 and 31 |
| Unsupported MSL | 447.2 | 0.6 | 3.6 and 5.2 |
| Q4 (mol%) | Q3 (mol%) | T3 (mol%) | T2 (mol%) | [CH3] (mmol g−1) | |
|---|---|---|---|---|---|
| 0 h | 42.5 | 11.0 | 34.2 | 12.3 | 7.1 |
| 248 h | 37.8 | 0.0 | 51.1 | 11.1 | 9.5 |
| Membranes | CM (wt%) | T (°C) | J (kg m−2 h−1) | α | Modifier | Refs. |
|---|---|---|---|---|---|---|
| PEBAX-4033 | 1.0 | 40 | 0.03 | 33.0 | -F | [57] |
| PERVAP-1060 | 1.0 | 40 | 0.70 | 270.0 | -F | [57] |
| PERVAP-1070 | 1.0 | 40 | 0.25 | 280.0 | -F | [57] |
| M10/MFFK | 1.0 | 50 | 0.82 | 310.0 | -CH3 | [16] |
| Al2O3-5nm | 1.0 | 35 | 0.70 | 1.1 | FAS | [20] |
| TiO2-5KD | 1.0 | 35 | 1.95 | 84.0 | FAS | [20] |
| ZrO2-5KD | 1.0 | 35 | 1.65 | 56.0 | FAS | [20] |
| ZrO2-3nm | 1.5 | 35 | 0.50 | 15.0 | FAS | [19] |
| ZrO2-200nm | 1.5 | 35 | 7.00 | 27.0 | FAS | [19] |
| (0.005PFOTES)SiO2 | 3.0 | 40 | 0.35 | 24.6 | FAS | [35] |
| (0.005TFPTES)SiO2 | 3.0 | 40 | 0.31 | 19.1 | FAS | [35] |
| (0.005OTES)SiO2 | 3.0 | 40 | 0.32 | 15.3 | OTES | [35] |
| (0.005PTES)SiO2 | 3.0 | 40 | 0.39 | 12.0 | PTES | [35] |
| MSL-10 | 4.6 | 30 | 0.45 | 27.1 | MTMS | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Wang, Y.; Li, Q.; Cui, S.; Tang, M.; Nie, Z.; Wei, Q. A Methyl-Modified Silica Layer Supported on Porous Ceramic Membranes for the Enhanced Separation of Methyl Tert-Butyl Ether from Aqueous Solution. Membranes 2022, 12, 452. https://doi.org/10.3390/membranes12050452
Xu L, Wang Y, Li Q, Cui S, Tang M, Nie Z, Wei Q. A Methyl-Modified Silica Layer Supported on Porous Ceramic Membranes for the Enhanced Separation of Methyl Tert-Butyl Ether from Aqueous Solution. Membranes. 2022; 12(5):452. https://doi.org/10.3390/membranes12050452
Chicago/Turabian StyleXu, Ligang, Yali Wang, Qunyan Li, Suping Cui, Mingxue Tang, Zuoren Nie, and Qi Wei. 2022. "A Methyl-Modified Silica Layer Supported on Porous Ceramic Membranes for the Enhanced Separation of Methyl Tert-Butyl Ether from Aqueous Solution" Membranes 12, no. 5: 452. https://doi.org/10.3390/membranes12050452
APA StyleXu, L., Wang, Y., Li, Q., Cui, S., Tang, M., Nie, Z., & Wei, Q. (2022). A Methyl-Modified Silica Layer Supported on Porous Ceramic Membranes for the Enhanced Separation of Methyl Tert-Butyl Ether from Aqueous Solution. Membranes, 12(5), 452. https://doi.org/10.3390/membranes12050452