The Effect of Solution Casting Temperature and Ultrasound Treatment on PEBAX MH-1657/ZIF-8 Mixed Matrix Membranes Morphology and Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
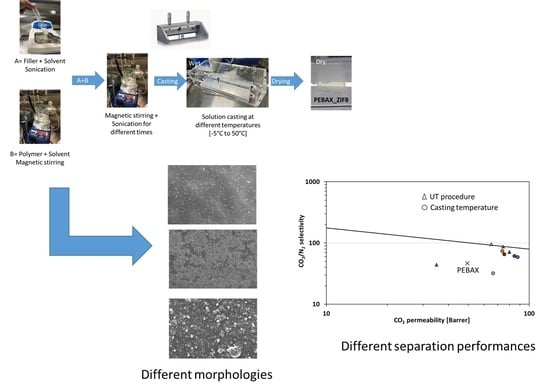
2.2. Membrane Preparation
2.3. Characterization
2.3.1. SEM (Scanning Electron Microscope)
2.3.2. XRD Experiment
2.3.3. DSC Experiment
2.3.4. AFM (Atomic Force Microscopy)
2.3.5. Gas Permeation
3. Results
3.1. Effect of Casting Temperature
3.1.1. Morphology of Neat PEBAX
3.1.2. Morphology of MMMs
3.1.3. Crystallinity
3.1.4. Separation Performances
Permeability and Selectivity
Diffusivity and Solubility
3.2. Effect of Ultrasound Treatement
3.2.1. Morphology
3.2.2. Separation Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abanades, J.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.; Li, H.; Ho, M.; Mangano, E.; Brandani, S. Emerging CO2 capture systems. Int. J. Greenh. Gas Control 2015, 40, 126–166. [Google Scholar] [CrossRef] [Green Version]
- Credence Research. Gas Separation Membranes Market by Type, by Application—Grouwth Future Prospects and Competitive Analysis, 2016–2024; Credence Research: San Jose, CA, USA, 2017. [Google Scholar]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the Cost of CO2 Capture from Flue Gases Using Membrane Technology. Ind. Eng. Chem. Res. 2008, 47, 1562–1568. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Jameh, A.A.; Mohammadi, T.; Bakhtiari, O. Preparation of PEBAX-1074/modified ZIF-8 nanoparticles mixed matrix membranes for CO2 removal from natural gas. Sep. Purif. Technol. 2020, 231, 115900. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic–inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 1–3, 87–98. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Peinemann, K.; Lai, Z. Carbon dioxide selective mixed matrix composite membrane contianing ZIF-7 nanofillers. J. Membr. Sci. 2013, 425–426, 235–242. [Google Scholar] [CrossRef]
- Etxeberria-Benavides, M.; David, O.; Johnson, T.; Łozińska, M.M.; Orsi, A.; Wright, P.A.; Mastel, S.; Hillenbrand, R.; Kapteijn, F.; Gascon, J. High performance mixed matrix membranes (MMMs) composed of ZIF-94 filler and 6FDA-DAM polymer. J. Membr. Sci. 2018, 550, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Nataraj, S.; Roussenova, M.V.; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A.; et al. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 2012, 5, 8359. [Google Scholar] [CrossRef]
- Smith, S.; Lau, C.; Mardel, J.; Kitchin, M.; Konstas, K.; Ladewig, B.; Hill, M. Physical aging in glassy mixed matrix membranes: Tuning particle interaction for mechanically robust nanocomposite films. J. Mater. Chem. A 2016, 4, 10627–10634. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Alberto, M.; Bhavsar, R.; Luque-Alled, J.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Impeded physical aging in PIM-1 membranes containing graphene-like fillers. J. Membr. Sci. 2018, 563, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Begni, F.; Paul, G.; Lasseuguette, E.; Mangano, E.; Bisio, C.; Ferrari, M.-C.; Gatti, G. Synthetic Saponite Clays as Additives for Reducing Aging Effects in PIM1 Membranes. ACS Appl. Polym. Mater. 2020, 2, 3481–3490. [Google Scholar] [CrossRef]
- Begni, F.; Lasseuguette, E.; Paul, G.; Bisio, C.; Marchese, L.; Gatti, G.; Ferrari, M.C. Hyper Cross-Linked Polymers as Additives for Preventing Aging of PIM-1 Membranes. Membranes 2021, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Gur, T. Permselectivity of zeolite filled polysulfone gas separation membranes. J. Membr. Sci. 1994, 93, 283–289. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Li, H.; Zhang, Y.; Chen, V. Improved operational stability of Pebax-based gas separation membranes with ZIF-8: A comparative study of flat sheet and composite hollow fibre membranes. J. Membr. Sci. 2017, 524, 266–279. [Google Scholar] [CrossRef]
- Bryan, N.; Lasseuguette, E.; van Dalen, M.; Permogorov, N.; Amieiro, A.; Brandani, S.; Ferrari, M.-C. Development of Mixed Matrix Membranes Containing Zeolites for Post-combustion Carbon Capture. Energy Procedia 2014, 63, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Azizi, N.; Mahdavi, H.; Isanejad, M.; Mohammadi, T. Effects of low and high molecular mass PEG incorporation into different types of poly(ether-b-amide) copolymers on the permeation properties of CO2 and CH4. J. Polym. Res. 2017, 24, 141. [Google Scholar] [CrossRef]
- Didden, J.; Thur, R.; Volodin, A.; Vankelecom, I. Blending PPO-based molecules with Pebax MH 1657 in membranes for gas separation. J. Appl. Polym. Sci. 2018, 135, 46433. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.; Janiak, C.; Sumby, C. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef]
- Adatoz, A.; Avci, S.; Kskin, S. Opportunities and challenges of MOF-based membranes in gas separations. Sep. Purif. Technol. 2015, 152, 207–237. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, L.; Wang, C.; Yu, J.; Zhnag, L.; Pan, Y. Enhanced permeation performance of polyether-polyamide block copolymer membranes through incorporating ZIF-8 nanocrystals. Chin. J. Chem. Eng. 2017, 25, 882–891. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.; Mohammadi, T.; Kargari, A. CO2/CH4 separation by high performance co-casted ZIF-8/Pebax 1657/PES mixed matrix membrane. J. Nat. Gas Sci. Eng. 2002, 21, 81–91. [Google Scholar]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Isanejad, M.; Azizi, N.; Mohammadi, T. Pebax membrane for CO2/CH4 separation: Effects of various solvents on morphology and performance. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Karamouz, F.; Magsoudi, H.; Yegani, R. Synthesis and characterization of high permeable PEBA membranes for CO2/CH4 separation. J. Nat. Gas Sci. Eng. 2016, 35, 980–985. [Google Scholar] [CrossRef]
- Martinez-Izquierdo, L.; Malankowska, M.; Sanchez-Lainez, J.; Tellez, C.; Coronas, J. Poly(ether-block-amide) copolymer membrane for CO2/N2 separation: The influence of the casting solution concentration on its morphology, thermal properties and gas separation performance. R. Soc. Open Sci. 2019, 6, 190866. [Google Scholar] [CrossRef] [Green Version]
- Wahab, M.; Sunarti, A.R. Influence of PVDF/Pebax TFC Casting Temperature towards CO2/N2 Gas Separation. Indian J. Sci. Technol. 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Wallinder, I.O. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J. Nanopart. Res. 2016, 18, 285. [Google Scholar] [CrossRef] [Green Version]
- QSonica. Proven Nanoparticle Dispersion Technology. Available online: https://www.sonicator.com/pages/nanoparticle-dispersion (accessed on 6 February 2022).
- Sabnis, S.S.; Gogate, P.R. Ultrasound assisted size reduction of DADPS based on recrystallization. Ultrason. Sonochem. 2019, 54, 198–209. [Google Scholar] [CrossRef]
- Ahmad, M.; Sarfraz, M.; Ba-Shammakh, M.; Naseer, K.; Ahmed, M.A. Optimizing membrane synthesis parameters via Taguchi method: An approach to prepare high performance mixed-matrix membranes for carbon capture applications. Can. J. Chem. Eng. 2020, 100, 143–155. [Google Scholar] [CrossRef]
- Zheng, W.; Ding, R.; Yang, K.; Dai, Y.; Yan, X.; He, G. ZIF-8 nanoparticles with tunable size for enhanced CO2 capture of Pebax based MMMs. Sep. Purif. Technol. 2019, 214, 111–119. [Google Scholar] [CrossRef]
- Li, H.; Tuo, L.; Yang, K.; Jeong, H.-K.; Dai, Y.; He, G.; Zhao, W. Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: Interfacial toughening effect of ionic liquid. J. Membr. Sci. 2016, 511, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Nafisi, V.; Hagg, M.-B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Membr. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Rahman, M.; Shishastskiy, S.; Abetz, C.; Georgopanos, P.; Neumann, S.; Khan, M.M.; Filiz, V.; Abetz, V. Influence of temperature upon properties of tailor-made PEBAXs MH 1657 nanocomposite membranes for post-combustion CO2 capture. J. Membr. Sci. 2014, 469, 344–354. [Google Scholar] [CrossRef] [Green Version]
- Dietz, W. Effect of cooling on crystallization and microstructure of polypropylene. Polym. Eng. Sci. 2016, 56, 1291–1302. [Google Scholar] [CrossRef]
- Che, J.; Burger, C.; Toki, S.; Rong, L.; Hsiao, B.S.; Amnuaypornsri, S.; Sakdapipanich, J. Crystal and Crystallites Structure of Natural Rubber and Peroxide-Vulcanized Natural Rubber by a Two-Dimensional Wide-Angle X-ray Diffraction Simulation Method. II. Strain-Induced Crystallization versus Temperature-Induced Crystallization. Macromolecules 2013, 46, 9712–9721. [Google Scholar] [CrossRef]
- Zhang, Y.; Tong, Y.; Li, X.; Guo, S.; Zhang, H.; Chen, X.; Cai, K.; Cheng, L.; He, W. Pebax Mixed-Matrix Membrane with Highly Dispersed ZIF-8@CNTs to Enhance CO2 /N2 Separation. ACS Omega 2021, 6, 18566–18575. [Google Scholar] [CrossRef]
- Meskhat, S.; Kaliaguine, S.; Rodrigue, D. Comparison between ZIF-67 and ZIF-8 in PEBAX MH-1657 mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2020, 235, 116150. [Google Scholar] [CrossRef]
- Ando, S.; Sato, S.; Nagai, K. Gas Permeation and Barrier Properties of Liquid Crystalline Polymers. In Liquid Crystalline Polymers; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 523–543. [Google Scholar]
- Sabetghadam, A.; Seoane, B.; Keskin, D.; Duim, N.; Rodenas, T.; Shahid, S.; Sorribas, S.; le Guillouzer, C.; Clet, G.; Tellez, C.; et al. Metal Organic Framework Crystals in Mixed-Matrix Membranes: Impact of the Filler Morphology on the Gas Separation Performance. Adv. Funct. Mater. 2016, 26, 3154–3163. [Google Scholar] [CrossRef]











| Materials | Permeability CO2 (Barrer) | Selectivity CO2/N2 | Reference |
|---|---|---|---|
| PEBAX | 49 (±3) | 47 (±5) | Our work |
| ZIF-8 | 850 | 1.7 | [43] |
| PEBAX_10%wtZIF-8 | 81 | 50 | Calculated from Maxwell equation |
| Materials | CO2 Permeability (Barrer) | CO2/N2 Selectivity | Measurement Conditions |
|---|---|---|---|
| PEBAX (This work) | 49 | 47 | 1.2 bar and 25 °C |
| MMM_4hC (This work) | 80 | 58 | 1.2 bar and 25 °C |
| MMM_50 °C (This work) | 84 | 62 | 1.2 bar and 25 °C |
| PEBAX [41] | 70 | 50 | 11 bar and 35 °C |
| PEBAX_ZIF8-5% [41] | 130 | 46 | 11 bar and 35 °C |
| PEBAX_ZIF67-5% [41] | 162 | 81 | 11 bar and 35 °C |
| PEBAX [34] | 75 | 45 | 1 bar and 20 °C |
| PEBAX_ZIF8-10% [34] | 120 | 52 | 1 bar and 20 °C |
| PEBAX [7] | 70 | 34 | 3.75 bar and 25 °C |
| PEBAX_ZIF7-8% [7] | 145 | 68 | 3.75 bar and 25 °C |
| PEBAX [16] | 120 | 47 | 3 bar and 25 °C |
| PEBAX_ZIF8-10% [16] | 175 | 41 | 3 bar and 25 °C |
| PEBAX [43] | 45 | 60 | 1.2 bar and 25 °C |
| PEBAX_ZIF94-25% [43] | 59 | 53 | 1.2 bar and 25 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasseuguette, E.; Fielder-Dunton, L.; Jian, Q.; Ferrari, M.-C. The Effect of Solution Casting Temperature and Ultrasound Treatment on PEBAX MH-1657/ZIF-8 Mixed Matrix Membranes Morphology and Performance. Membranes 2022, 12, 584. https://doi.org/10.3390/membranes12060584
Lasseuguette E, Fielder-Dunton L, Jian Q, Ferrari M-C. The Effect of Solution Casting Temperature and Ultrasound Treatment on PEBAX MH-1657/ZIF-8 Mixed Matrix Membranes Morphology and Performance. Membranes. 2022; 12(6):584. https://doi.org/10.3390/membranes12060584
Chicago/Turabian StyleLasseuguette, Elsa, Louise Fielder-Dunton, Qian Jian, and Maria-Chiara Ferrari. 2022. "The Effect of Solution Casting Temperature and Ultrasound Treatment on PEBAX MH-1657/ZIF-8 Mixed Matrix Membranes Morphology and Performance" Membranes 12, no. 6: 584. https://doi.org/10.3390/membranes12060584
APA StyleLasseuguette, E., Fielder-Dunton, L., Jian, Q., & Ferrari, M. -C. (2022). The Effect of Solution Casting Temperature and Ultrasound Treatment on PEBAX MH-1657/ZIF-8 Mixed Matrix Membranes Morphology and Performance. Membranes, 12(6), 584. https://doi.org/10.3390/membranes12060584