Studies of Protein Wastes Adsorption by Chitosan-Modified Nanofibers Decorated with Dye Wastes in Batch and Continuous Flow Processes: Potential Environmental Applications
Abstract
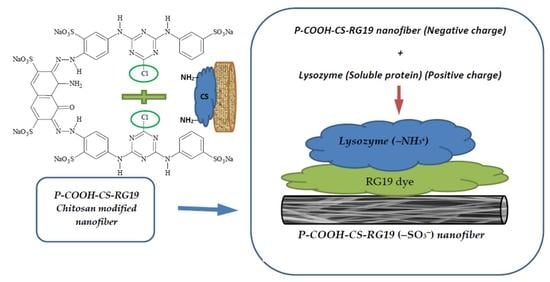
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Dyed Nanofiber Membrane
2.3. Characterization of Nanofiber Membranes
2.4. Lysozyme Removal Study
2.5. Lysozyme Desorption Study
2.6. Adsorption of Lysozyme in Flow Process
2.7. Continuous Removal of Lysozyme in Flow Process
2.8. Kinetic Models
2.9. Equilibrium Isotherm Models
2.10. Dynamic Binding Capacity
2.11. Dynamic Adsorption Performance Indicator
3. Results and Discussion
3.1. Physical and Chemical Properties of the Nanofibers
3.2. Operating Parameters for the Adsorption of Lysozyme
3.2.1. Coupling with Different Molecular Weights and pH of Chitosan
3.2.2. Coupling Concentration of Chitosan
3.3. Immobilization Parameters of Dye
3.3.1. Immobilization pH
3.3.2. Initial Dye Concentration
3.4. Lysozyme Adsorption by Dyed Nanofiber Membrane
3.4.1. Adsorption pH
3.4.2. Kinetic Study
3.4.3. Thermodynamic Study
3.5. Lysozyme Elution
3.6. Continuous Flow Process
3.7. Remarks of the Applications of the Nanofiber Used in This Work
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blackburn, R.S. Natural polysaccharides and their interactions with dye molecules: Applications in effluent treatment. Environ. Sci. Technol. 2004, 38, 4905–4909. [Google Scholar] [CrossRef] [PubMed]
- Donia, A.M.; Atia, A.A.; Al-Amrani, W.A.; El-Nahas, A.M. Effect of structural properties of acid dyes on their adsorption behaviour from aqueous solutions by amine modified silica. J. Hazard. Mater. 2009, 161, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Ortiz, H.I.; Puente-Urbina, B.; Mercado-Silva, J.A.; García-Uriostegui, L. Adsorption performance of mesoporous silicas towards a cationic dye. Influence of mesostructure on adsorption capacity. Int. J. Appl. Ceram. Technol. 2019, 16, 1533–1543. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kunacheva, C.; Stuckey, D.C. Analytical methods for soluble microbial products (SMP) and extracellular polymers (ECP) in wastewater treatment systems: A review. Water Res. 2014, 61, 1–18. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Ooi, C.-W.; Ling, T.C.; Shu-Jen, C.; Chen, S.-Y.; Chang, Y.-K. Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes. Clean Technol. Environ. Policy 2020, 22, 773–786. [Google Scholar] [CrossRef]
- Dizge, N.; Tansel, B.; Sizirici, B. Process intensification with a hybrid system: A tubular packed bed bioreactor with immobilized activated sludge culture coupled with membrane filtration. Chem. Eng. Process. Process Intensif. 2011, 50, 766–772. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chai, W.S.; Chen, S.-J.; Shih, J.-Y.; Koyande, A.K.; Liu, B.-L.; Chang, Y.-K. Removal of soluble microbial products and dyes using heavy metal wastes decorated on eggshell. Chemosphere 2021, 270, 128615. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Chu, L.; Tsai, J.-C.; Chiu, S.-J. Kinetic study of immobilized lysozyme on the extrudate-shaped NaY zeolite. Process. Biochem. 2006, 41, 1864–1874. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Huang, R.-Z.; Lin, S.-Y.; Chiu, S.-J.; Tsai, J.-C. Equilibrium study of immobilized lysozyme on the extrudate-shaped NaY zeolite. Biochem. Eng. J. 2006, 28, 1–9. [Google Scholar] [CrossRef]
- Cheng, T.-H.; Sankaran, R.; Show, P.L.; Ooi, C.W.; Liu, B.-L.; Chai, W.S.; Chang, Y.-K. Removal of protein wastes by cylinder-shaped NaY zeolite adsorbents decorated with heavy metal wastes. Int. J. Biol. Macromol. 2021, 185, 761–772. [Google Scholar] [CrossRef]
- Boyer, P.M.; Hsu, J.T. Effects of ligand concentration on protein adsorption in dye-ligand adsorbents. Chem. Eng. Sci. 1992, 47, 241–251. [Google Scholar] [CrossRef]
- Chen, K.-H.; Lee, S.-Y.; Show, P.-L.; Hong, S.-C.; Chang, Y.-K. Direct recovery of malate dehydrogenase from highly turbid yeast cell homogenate using dye-ligand affinity chromatography in stirred fluidized bed. J. Chromatogr. B 2018, 1100, 65–75. [Google Scholar] [CrossRef]
- Sheng-Shih Wang, S.; Yang, S.-M.; Hsin, A.; Chang, Y.-K. Dye-affinity nanofibrous membrane for adsorption of lysozyme: Preparation and performance evaluation. Food Technol. Biotechnol. 2018, 56, 40–50. [Google Scholar]
- Ng, I.S.; Song, C.P.; Ooi, C.W.; Tey, B.T.; Lee, Y.-H.; Chang, Y.-K. Purification of lysozyme from chicken egg white using nanofiber membrane immobilized with Reactive Orange 4 dye. Int. J. Biol. Macromol. 2019, 134, 458–468. [Google Scholar] [CrossRef]
- Show, P.L.; Ooi, C.W.; Lee, X.J.; Yang, C.-L.; Liu, B.-L.; Chang, Y.-K. Batch and dynamic adsorption of lysozyme from chicken egg white on dye-affinity nanofiber membranes modified by ethylene diamine and chitosan. Int. J. Biol. Macromol. 2020, 162, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-X.; Ooi, C.W.; Liu, B.-L.; Song, C.P.; Chiu, C.-Y.; Wang, C.-Y.; Chang, Y.-K. Antibacterial efficacy of poly (hexamethylene biguanide) immobilized on chitosan/dye-modified nanofiber membranes. Int. J. Biol. Macromol. 2021, 181, 508–520. [Google Scholar] [CrossRef]
- Smith, S.C.; Ahmed, F.; Gutierrez, K.M.; Rodrigues, D.F. A comparative study of lysozyme adsorption with graphene, graphene oxide, and single-walled carbon nanotubes: Potential environmental applications. Chem. Eng. J. 2014, 240, 147–154. [Google Scholar] [CrossRef]
- Cai, Z.; Song, X.; Zhang, Q.; Zhai, T. Electrospun polyindole nanofibers as a nano-adsorbent for heavy metal ions adsorption for wastewater treatment. Fibers Polym. 2017, 18, 502–513. [Google Scholar] [CrossRef]
- Lou, T.; Yan, X.; Wang, X. Chitosan coated polyacrylonitrile nanofibrous mat for dye adsorption. Int. J. Biol. Macromol. 2019, 135, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Shahrousvand, M.; Shojaei, S.; Khonakdar, H.A.; Asefnejad, A.; Goodarzi, V. Preparation of superabsorbent eco-friendly semi-interpenetrating network based on cross-linked poly acrylic acid/xanthan gum/graphene oxide (PAA/XG/GO): Characterization and dye removal ability. Int. J. Biol. Macromol. 2020, 152, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Suen, S.-Y.; TSAI, Y.-D. Comparison of ligand density and protein adsorption on dye-affinity membranes using different spacer arms. Sep. Sci. Technol. 2000, 35, 69–87. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.-L.; Ng, I.S.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-C.; Lin, W.-C. Surface modification and blood compatibility of polyacrylonitrile membrane with immobilized chitosan–heparin conjugate. J. Polym. Res. 2002, 9, 201–206. [Google Scholar] [CrossRef]
- Huong, D.T.M.; Liu, B.-L.; Chai, W.S.; Show, P.L.; Tsai, S.-L.; Chang, Y.-K. Highly efficient dye removal and lysozyme purification using strong and weak cation-exchange nanofiber membranes. Int. J. Biol. Macromol. 2020, 165, 1410–1421. [Google Scholar] [CrossRef]
- Grasselli, M.; Camperi, S.A.; del Cañizo, A.A.N.; Cascone, O. Direct lysozyme separation from egg white by dye membrane affinity chromatography. J. Sci. Food Agric. 1999, 79, 333–339. [Google Scholar] [CrossRef]
- Lee, P.-X.; Liu, B.-L.; Show, P.L.; Ooi, C.W.; Chai, W.S.; Munawaroh, H.S.H.; Chang, Y.-K. Removal of calcium ions from aqueous solution by bovine serum albumin (BSA)-modified nanofiber membrane: Dynamic adsorption performance and breakthrough analysis. Biochem. Eng. J. 2021, 171, 108016. [Google Scholar] [CrossRef]
- Arnold, F.; Blanch, H.; Wilke, C. Analysis of affinity separations: I: Predicting the performance of affinity adsorbers. Chem. Eng. J. 1985, 30, B9–B23. [Google Scholar] [CrossRef]
- Tsai, A.M.; Englert, D.; Graham, E.E. Study of the dynamic binding capacity of two anion exchangers using bovine serum albumin as a model protein. J. Chromatogr. A 1990, 504, 89–95. [Google Scholar] [CrossRef]
- Finette, G.M.; Mao, Q.M.; Hearn, M.T. Examination of protein adsorption in fluidized bed and packed bed columns at different temperatures using frontal chromatographic methods. Biotechnol. Bioeng. 1998, 58, 35–46. [Google Scholar] [CrossRef]
- Fahrner, R.; Iyer, H.; Blank, G. The optimal flow rate and column length for maximum production rate of protein A affinity chromatography. Bioprocess Eng. 1999, 21, 287–292. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Orr, V.; Zhong, L.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Babu, B. Modeling, simulation, and experimental validation for continuous Cr (VI) removal from aqueous solutions using sawdust as an adsorbent. Bioresour. Technol. 2009, 100, 5633–5640. [Google Scholar] [CrossRef]
- Onyango, M.S.; Leswifi, T.Y.; Ochieng, A.; Kuchar, D.; Otieno, F.O.; Matsuda, H. Breakthrough analysis for water defluoridation using surface-tailored zeolite in a fixed bed column. Ind. Eng. Chem. Res. 2009, 48, 931–937. [Google Scholar] [CrossRef]
- Kim, B.M.; Sircar, S. Adsorption characteristics of volatile anesthetics on activated carbons and performance of carbon canisters. Anesthesiology 1977, 46, 159–165. [Google Scholar] [CrossRef]
- Tariq, M.; Farooq, U.; Athar, M.; Salman, M.; Tariq, M. Biosorption of Cu (II) from aqueous solution onto immobilized Ficus religiosa branch powder in a fixed bed column: Breakthrough curves and mathematical modeling. Korean J. Chem. Eng. 2019, 36, 48–55. [Google Scholar] [CrossRef]
- Ang, T.N.; Young, B.R.; Taylor, M.; Burrell, R.; Aroua, M.K.; Baroutian, S. Breakthrough analysis of continuous fixed-bed adsorption of sevoflurane using activated carbons. Chemosphere 2020, 239, 124839. [Google Scholar] [CrossRef]
- Hu, A.; Ren, G.; Che, J.; Guo, Y.; Ye, J.; Zhou, S. Phosphate recovery with granular acid-activated neutralized red mud: Fixed-bed column performance and breakthrough curve modelling. J. Environ. Sci. 2020, 90, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, Y.; Qiu, Y.; Wang, X.; Hu, Y.; Yang, J.; Cai, J.; Kennedy, J.F. A new green technology for direct production of low molecular weight chitosan. Carbohydr. Polym. 2008, 74, 127–132. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.-C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Choi, J.J.; Le, Q.-V.; Kim, D.; Kim, Y.B.; Shim, G.; Oh, Y.-K. High molecular weight chitosan-complexed RNA nanoadjuvant for effective cancer immunotherapy. Pharmaceutics 2019, 11, 680. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Lu, J.; Cui, Z. Separation of lysozyme from chicken egg white using ultrafiltration. Sep. Purif. Technol. 2006, 48, 133–142. [Google Scholar] [CrossRef]
- Uygun, M.; Akduman, B.; Uygun, D.A.; Akgöl, S.; Denizli, A. Dye functionalized cryogel columns for reversible lysozyme adsorption. J. Biomater. Sci. Polym. Ed. 2015, 26, 277–289. [Google Scholar] [CrossRef]
- Weber Jr, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Ofomaja, A.E. Intraparticle diffusion process for lead (II) biosorption onto mansonia wood sawdust. Bioresour. Technol. 2010, 101, 5868–5876. [Google Scholar] [CrossRef]
- Li, T.-t.; Liu, Y.-g.; Peng, Q.-q.; Hu, X.-j.; Liao, T.; Wang, H.; Lu, M. Removal of lead (II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem. Eng. J. 2013, 214, 189–197. [Google Scholar] [CrossRef]
- Lallai, A.; Mura, G.; Palmas, S.; Polcaro, A.M.; Baraccani, L. Degradation of para-hydroxybenzoic acid by means of mixed microbial cultures. Environ. Sci. Pollut. Res. 2003, 10, 221–224. [Google Scholar] [CrossRef]
- Nataraj, S.; Yang, K.; Aminabhavi, T. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Cheng, H.-I.; Ooi, C.W.; Song, C.P.; Liu, B.-L. Adsorption and purification performance of lysozyme from chicken egg white using ion exchange nanofiber membrane modified by ethylene diamine and bromoacetic acid. Food Chem. 2021, 358, 129914. [Google Scholar] [CrossRef]
- Hashim, M.A.; Chu, K.H.; Tsan, P.S. Effects of ionic strength and pH on the adsorption equilibria of lysozyme on ion exchangers. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 1995, 62, 253–260. [Google Scholar] [CrossRef]
- Kaçar, Y.; Arica, M.Y. Procion Green H-E4BD-immobilized porous poly (hydroxyethylmethacrylate) ion-exchange membrane: Preparation and application to lysozyme adsorption. Colloids Surf. B Biointerfaces 2001, 22, 227–236. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Yılmaz, M.; Yalçın, E.; Bayramoğlu, G. Affinity membrane chromatography: Relationship of dye-ligand type to surface polarity and their effect on lysozyme separation and purification. J. Chromatogr. B 2004, 805, 315–323. [Google Scholar] [CrossRef]
- Arıca, M.Y.; Bayramoğlu, G. Purification of lysozyme from egg white by Reactive Blue 4 and Reactive Red 120 dye-ligands immobilised composite membranes. Process. Biochem. 2005, 40, 1433–1442. [Google Scholar] [CrossRef]
- Yılmaz, M.; Bayramoǧlu, G.; Arıca, M.Y. Separation and purification of lysozyme by Reactive Green 19 immobilised membrane affinity chromatography. Food Chem. 2005, 89, 11–18. [Google Scholar] [CrossRef]
- Liu, Y.C.; Stellwagen, E. Zonal chromatographic analysis of the interaction of alcohol dehydrogenase with blue-sepharose. J. Chromatogr. B Biomed. Sci. Appl. 1986, 376, 149–155. [Google Scholar] [CrossRef]
- Chen, T.-X.; Nie, H.-L.; Li, S.-B.; Branford-White, C.; Su, S.-N.; Zhu, L.-M. Comparison: Adsorption of papain using immobilized dye ligands on affinity membranes. Colloids Surf. B Biointerfaces 2009, 72, 25–31. [Google Scholar] [CrossRef]
- Liu, B.-L.; Ooi, C.W.; Ng, I.-S.; Show, P.L.; Lin, K.-J.; Chang, Y.-K. Effective purification of lysozyme from chicken egg white by tris (hydroxymethyl) aminomethane affinity nanofiber membrane. Food Chem. 2020, 327, 127038. [Google Scholar] [CrossRef]













| Membranes | μmol−COOH/g Membrane | μmol−NH2/g Membrane |
|---|---|---|
| P-COOH | 261.66 | - |
| P-COOH-CS (50 kDa) | 0.87 | 179.06 |
| P-COOH-CS (210 kDa) | 1.43 | 145.39 |
| P-COOH-CS (340 kDa) | 7.65 | 112.65 |
| Lysozyme (mg/mL) | Pseudo-First-Order | Pseudo-Second-Order | Elovich | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 (mg/g·min1/2) | q1 (mg/g) | R2 | k1 | qcal (mg/g) | qexp (mg/g) | R2 | α | β | R2 | |
| 0.5 | 0.0273 | 176.35 | 0.5794 | 1.54 × 10−3 | 181.82 | 185.60 | 0.9981 | 907.5200 | 0.04780 | 0.7741 |
| 1.0 | 0.0285 | 356.48 | 0.9092 | 4.61 × 10−4 | 370.37 | 367.69 | 0.9970 | 342.2725 | 0.01855 | 0.8838 |
| 2.0 | 0.0271 | 598.66 | 0.9455 | 1.80 × 10−4 | 625.12 | 610.90 | 0.9963 | 183.6610 | 0.00892 | 0.9243 |
| Lysozyme (mg/mL) | Intraparticle Diffusion Model | |||||
|---|---|---|---|---|---|---|
| First Stage | Second Stage | Third Stage | ||||
| kp (mg/g∙min1/2) | R2 | kp (mg/g∙min1/2) | R2 | kp (mg/g∙min1/2) | R2 | |
| 0.5 | 32.73 | 0.9945 | 8.312 | 0.7691 | 1.165 | 0.2390 |
| 1.0 | 55.59 | 0.9940 | 18.52 | 0.9134 | 2.581 | 0.4035 |
| 2.0 | 82.14 | 0.9943 | 23.15 | 0.9506 | 5.534 | 0.6602 |
| Temperature | Langmuir | Freundlich | Temkin isotherm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (K) | qmax (mg/g) | Kd, (mg/mL) | R2 | nF | Kd,F | R2 | b | KT (mg/mL) | R2 |
| 288 | 648.5084 | 0.01309 | 0.9973 | 38.1679 | 669.1444 | 0.1132 | 144.9414 | 4.0832 × 1017 | 0.1174 |
| 298 | 708.7172 | 0.05489 | 0.9881 | 4.8685 | 660.5018 | 0.7697 | 30.1041 | 2874.2104 | 0.8537 |
| 318 | 1293.6610 | 0.07280 | 0.9964 | 7.2254 | 1207.1288 | 0.8820 | 17.4281 | 2853.9671 | 0.8729 |
| Temperature | Kd | Kads | ΔG0ads | ΔH0ads | ΔS0ads |
|---|---|---|---|---|---|
| (K) | (mg/mL) | (mL/mg) | (kJ/mol) | (kJ/mol) | (kJ/mol) |
| 288 | 0.01309 | 76.3941 | −10382.0337 | −39641.1520 | 10244.3909 |
| 298 | 0.05489 | 18.2182 | −7190.9646 | 7057.9407 | |
| 318 | 0.07280 | 13.7362 | −6926.9962 | 6802.3385 |
| Membranes | Maximum Binding Capacity (mg/g) | Maximum Binding Capacity (mg/cm2) | References |
|---|---|---|---|
| P-COOH-CS-RG19 | 769.23 | 4.70 | This work |
| PAN-COOH-CS-RB2 nanofiber membrane | 245.58 | 1.50 | [14] |
| P-COOH-CS-RO4 nanofiber membrane | 369.92 | 2.26 | [15] |
| P-COOH-EDA-RB49 nanofiber membrane | 587.25 | 3.59 | [16] |
| P-COOH-CS-RB49 nanofiber membrane | 416.67 | 2.54 | [16] |
| Reactive Blue 4 immobilized onto pHEMA/chitosan membranes. | 21.67 | 1.64 | [54] |
| Reactive Red 120 immobilized pHEMA/chitosan composite membrane | 31.75 | 2.40 | [54] |
| Procion Green H4-G immobilized pHEMA/chitosan composite membrane | 63.66 | 4.81 | [53] |
| Procion Brown MX-5BR immobilized pHEMA/chitosan composite membrane | 48.06 | 3.63 | [53] |
| Procion Brown MX-5BR immobilized pHEMA/chitosan IPNs membrane | 101.75 | 3.33 | [52] |
| Procion Green H-E4BD immobilized pHEMA ion exchange membrane | 11.22 | 0.42 | [51] |
| Reactive Green 19 immobilized pHEMA/chitosan composite membrane | 48.25 | 1.97 | [55] |
| Procion Brown MX-5BR immobilized pHEMA/chitosan composite membrane | 96.43 | 3.16 | [56] |
| Procion Green H-4G immobilized pHEMA membrane | 63.65 | 2.19 | [57] |
| P-COOH-Tris nanofiber membrane | 345.83 | 2.11 | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, D.-L.; Thanh, D.T.H.; Show, P.-L.; How, S.-C.; Chiu, C.-Y.; Hsu, M.; Chia, S.R.; Chen, K.-H.; Chang, Y.-K. Studies of Protein Wastes Adsorption by Chitosan-Modified Nanofibers Decorated with Dye Wastes in Batch and Continuous Flow Processes: Potential Environmental Applications. Membranes 2022, 12, 759. https://doi.org/10.3390/membranes12080759
Cai D-L, Thanh DTH, Show P-L, How S-C, Chiu C-Y, Hsu M, Chia SR, Chen K-H, Chang Y-K. Studies of Protein Wastes Adsorption by Chitosan-Modified Nanofibers Decorated with Dye Wastes in Batch and Continuous Flow Processes: Potential Environmental Applications. Membranes. 2022; 12(8):759. https://doi.org/10.3390/membranes12080759
Chicago/Turabian StyleCai, Dai-Lun, Dinh Thi Hong Thanh, Pau-Loke Show, Su-Chun How, Chen-Yaw Chiu, Michael Hsu, Shir Reen Chia, Kuei-Hsiang Chen, and Yu-Kaung Chang. 2022. "Studies of Protein Wastes Adsorption by Chitosan-Modified Nanofibers Decorated with Dye Wastes in Batch and Continuous Flow Processes: Potential Environmental Applications" Membranes 12, no. 8: 759. https://doi.org/10.3390/membranes12080759
APA StyleCai, D. -L., Thanh, D. T. H., Show, P. -L., How, S. -C., Chiu, C. -Y., Hsu, M., Chia, S. R., Chen, K. -H., & Chang, Y. -K. (2022). Studies of Protein Wastes Adsorption by Chitosan-Modified Nanofibers Decorated with Dye Wastes in Batch and Continuous Flow Processes: Potential Environmental Applications. Membranes, 12(8), 759. https://doi.org/10.3390/membranes12080759