Membrane Technologies for Nitrogen Recovery from Waste Streams: Scientometrics and Technical Analysis
Abstract
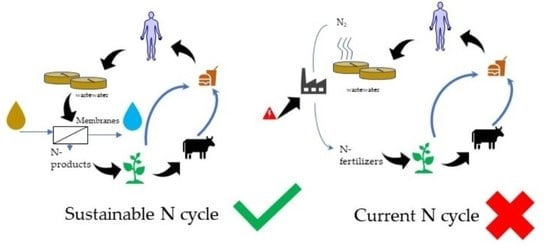
:1. Introduction
2. Literature Analysis
3. Nitrogen Recovery Waste Streams
4. Pressure-Driven Membrane Processes
5. Thermally-Driven Membrane Processes
6. Osmotically-Driven Membrane Processes
7. Biologically-Enhanced Membrane (BES) Processes
7.1. AnMBR
7.2. OMBR
7.3. Photobioreactor Membranes (PBRMs)
8. Electro-Chemical Membrane Processes (ECMs)
9. Gas Permeable Membranes (GPMs)
10. Effective Hybrid Membrane Systems for Nitrogen Recovery
11. Outlook for Realistic Research Development
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beckinghausen, A.; Odlare, M.; Thorin, E.; Schwede, S. From removal to recovery: An evaluation of nitrogen recovery techniques from wastewater. Appl. Energy 2020, 263, 114616. [Google Scholar] [CrossRef]
- Vineyard, D.; Hicks, A.; Karthikeyan, K.; Davidson, C.; Barak, P. Life cycle assessment of electrodialysis for sidestream nitrogen recovery in municipal wastewater treatment. Clean. Environ. Syst. 2021, 2, 100026. [Google Scholar] [CrossRef]
- Yan, T.; Ye, Y.; Ma, H.; Zhang, Y.; Guo, W.; Du, B.; Wei, Q.; Wei, D.; Ngo, H.H. A critical review on membrane hybrid system for nutrient recovery from wastewater. Chem. Eng. J. 2018, 348, 143–156. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An electrochemical haber-bosch process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.; Erisman, J.; Abrol, Y.; Bekunda, M.; Datta, A.; Davidson, E.; De Vries, W.; Oenema, O. Our Nutrient World. The Challenge to Produce More Food & Energy with Less Pollution; Centre for Ecology & Hydrology: Edinburgh, UK, 2013. [Google Scholar]
- Su, B.; Lin, Y.; Wang, J.; Quan, X.; Chang, Z.; Rui, C. Sewage treatment system for improving energy efficiency based on particle swarm optimization algorithm. Energy Rep. 2022, 8, 8701–8708. [Google Scholar] [CrossRef]
- Nowak, O. Benchmarks for the energy demand of nutrient removal plants. Water Sci. Technol. 2003, 47, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, H.; Yin, Y.; Zeng, F.; Cui, Z. Smart energy savings for aeration control in wastewater treatment. Energy Rep. 2022, 8, 1711–1721. [Google Scholar] [CrossRef]
- International Energy Agency. Water Energy Nexus; OECD/IEA: Paris, France, 2016. [Google Scholar]
- Righetto, I.; Al-Juboori, R.A.; Kaljunen, J.U.; Huynh, N.; Mikola, A. Nitrogen Recovery from Landfill Leachate Using Lab- and Pilot-Scale Membrane Contactors: Research into Fouling Development and Membrane Characterization Effects. Membranes 2022, 12, 837. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Liu, Q.; Bui, X.T.; Hoang, N.B. Bio-membrane integrated systems for nitrogen recovery from wastewater in circular bioeconomy. Chemosphere 2022, 289, 133175. [Google Scholar] [CrossRef]
- Song, X.; Luo, W.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. Resource recovery from wastewater by anaerobic membrane bioreactors: Opportunities and challenges. Bioresour. Technol. 2018, 270, 669–677. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward osmosis as a platform for resource recovery from municipal wastewater-A critical assessment of the literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Ang, W.L.; Mohammad, A.W.; Johnson, D.; Hilal, N. Forward osmosis research trends in desalination and wastewater treatment: A review of research trends over the past decade. J. Water Process Eng. 2019, 31, 100886. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Kurniawan, T.A.; Puteh, M.H.; Ismail, A.; Khongnakorn, W.; Rahman, M.A.; Jaafar, J. Advances in adsorptive membrane technology for water treatment and resource recovery applications: A critical review. J. Environ. Chem. Eng. 2022, 10, 107633. [Google Scholar] [CrossRef]
- Voortman, W.; Simpson, A.; Kerr, C.; Buckley, C. Application of electrochemical membrane processes to the treatment of aqueous effluent streams. Water Sci. Technol. 1992, 25, 329–337. [Google Scholar] [CrossRef]
- Bilstad, T. Nitrogen separation from domestic wastewater by reverse osmosis. J. Membr. Sci. 1995, 102, 93–102. [Google Scholar] [CrossRef]
- Chen, T.; Ni, C.; Chen, J.; Lin, J. High-strength nitrogen removal of opto-electronic industrial wastewater in membrane bioreactor-a pilot study. Water Sci. Technol. 2003, 48, 191–198. [Google Scholar] [CrossRef]
- Pieters, J.; Neukermans, G.; Colanbeen, M. Farm-scale membrane filtration of sow slurry. J. Agric. Eng. Res. 1999, 73, 403–409. [Google Scholar] [CrossRef]
- Grundestam, J.; Hellström, D. Wastewater treatment with anaerobic membrane bioreactor and reverse osmosis. Water Sci. Technol. 2007, 56, 211–217. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Impact of the Ukraine-Russia Conflict on Global Food Security and Related Matters under the Mandate of the Food and Agriculture Organization of the United Nations (FAO); FAO: Rome, Italy, 2022; pp. 1–17. [Google Scholar]
- Courtney, C.; Randall, D.G. Concentrating stabilized urine with reverse osmosis: How does stabilization method and pre-treatment affect nutrient recovery, flux, and scaling? Water Res. 2022, 209, 117970. [Google Scholar] [CrossRef]
- Uzkurt Kaljunen, J.; Al-Juboori, R.A.; Mikola, A.; Righetto, I.; Konola, I. Newly developed membrane contactor-based N and P recovery process: Pilot-scale field experiments and cost analysis. J. Clean. Prod. 2021, 281, 125288. [Google Scholar] [CrossRef]
- Guo, J.; Lee, J.-G.; Tan, T.; Yeo, J.; Wong, P.W.; Ghaffour, N.; An, A.K. Enhanced ammonia recovery from wastewater by Nafion membrane with highly porous honeycomb nanostructure and its mechanism in membrane distillation. J. Membr. Sci. 2019, 590, 117265. [Google Scholar] [CrossRef]
- Noriega-Hevia, G.; Serralta, J.; Borrás, L.; Seco, A.; Ferrer, J. Nitrogen recovery using a membrane contactor: Modelling nitrogen and pH evolution. J. Environ. Chem. Eng. 2020, 8, 103880. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Kaljunen, J.U.; Righetto, I.; Mikola, A. Membrane contactor onsite piloting for nutrient recovery from mesophilic digester reject water: The effect of process conditions and pre-treatment options. Sep. Purif. Technol. 2022, 303, 122250. [Google Scholar] [CrossRef]
- Van der Hoek, J.P.; Duijff, R.; Reinstra, O. Nitrogen recovery from wastewater: Possibilities, competition with other resources, and adaptation pathways. Sustainability 2018, 10, 4605. [Google Scholar] [CrossRef] [Green Version]
- Lauterböck, B.; Ortner, M.; Haider, R.; Fuchs, W. Counteracting ammonia inhibition in anaerobic digestion by removal with a hollow fiber membrane contactor. Water Res. 2012, 46, 4861–4869. [Google Scholar] [CrossRef]
- Shin, C.; Szczuka, A.; Jiang, R.; Mitch, W.A.; Criddle, C.S. Optimization of reverse osmosis operational conditions to maximize ammonia removal from the effluent of an anaerobic membrane bioreactor. Environ. Sci. Water Res. Technol. 2021, 7, 739–747. [Google Scholar] [CrossRef]
- Woo, Y.C.; Lee, J.K.; Kim, H.-S. Fouling characteristics of microfiltration membranes by organic and inorganic matter and evaluation of flux recovery by chemical cleaning. Desalin. Water Treat. 2014, 52, 6920–6929. [Google Scholar] [CrossRef]
- Daguerre-Martini, S.; Vanotti, M.B.; Rodriguez-Pastor, M.; Rosal, A.; Moral, R. Nitrogen recovery from wastewater using gas-permeable membranes: Impact of inorganic carbon content and natural organic matter. Water Res. 2018, 137, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Alkhatib, A.; Ayari, M.A.; Hawari, A.H. Fouling mitigation strategies for different foulants in membrane distillation. Chem. Eng. Process. Process Intensif. 2021, 167, 108517. [Google Scholar] [CrossRef]
- Lee, W.; An, S.; Choi, Y. Ammonia harvesting via membrane gas extraction at moderately alkaline pH: A step toward net-profitable nitrogen recovery from domestic wastewater. Chem. Eng. J. 2021, 405, 126662. [Google Scholar] [CrossRef]
- Gong, H.; Yan, Z.; Liang, K.; Jin, Z.; Wang, K. Concentrating process of liquid digestate by disk tube-reverse osmosis system. Desalination 2013, 326, 30–36. [Google Scholar] [CrossRef]
- Spiller, M.; Moretti, M.; De Paepe, J.; Vlaeminck, S.E. Environmental and economic sustainability of the nitrogen recovery paradigm: Evidence from a structured literature review. Resour. Conserv. Recycl. 2022, 184, 106406. [Google Scholar] [CrossRef]
- Sanchis-Perucho, P.; Aguado, D.; Ferrer, J.; Seco, A.; Robles, Á. Dynamic Membranes for Enhancing Resources Recovery from Municipal Wastewater. Membranes 2022, 12, 214. [Google Scholar] [CrossRef]
- Damirchia, M.; Koyuncua, I. Nutrient recovery from concentrated municipal wastewater by forward osmosis membrane and MgCl. In Proceedings of the 6th MEMTEK International Symposium on Membrane Technologies and Applications (MEMTEK 2019), Istanbul, Turkey, 18–20 November 2019; p. 20. [Google Scholar]
- Munasinghe-Arachchige, S.P.; Abeysiriwardana-Arachchige, I.S.A.; Delanka-Pedige, H.M.K.; Cooke, P.; Nirmalakhandan, N. Nitrogen-fertilizer recovery from urban sewage via gas permeable membrane: Process analysis, modeling, and intensification. Chem. Eng. J. 2021, 411, 128443. [Google Scholar] [CrossRef]
- Dow, N.; Saldin, T.F.; Duke, M.; Yang, X. Pilot demonstration of nitrogen removal from municipal wastewater by vacuum membrane distillation. J. Water Process Eng. 2022, 47, 102726. [Google Scholar] [CrossRef]
- Jung, M.; Oh, T.; Rhu, D.; Liberzon, J.; Kang, S.J.; Daigger, G.T.; Kim, S. A high-rate and stable nitrogen removal from reject water in a full-scale two-stage AMX® system. Water Sci. Technol. 2021, 83, 652–663. [Google Scholar] [CrossRef]
- Jimenez, J.; Bott, C.; Love, N.; Bratby, J. Source separation of urine as an alternative solution to nutrient management in biological nutrient removal treatment plants. Water Environ. Res. 2015, 87, 2120–2129. [Google Scholar] [CrossRef]
- Ray, H. Nitrogen Recovery from Human Urine by Membrane Processes. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, December 2020. [Google Scholar]
- Yu, C.; Yin, W.; Yu, Z.; Chen, J.; Huang, R.; Zhou, X. Membrane technologies in toilet urine treatment for toilet urine resource utilization: A review. RSC Adv. 2021, 11, 35525–35535. [Google Scholar] [CrossRef]
- Flanagan, C.; Randall, D. Development of a novel nutrient recovery urinal for on-site fertilizer production. J. Environ. Chem. Eng. 2018, 6, 6344–6350. [Google Scholar] [CrossRef]
- Ikematsu, M.; Kaneda, K.; Iseki, M.; Yasuda, M. Electrochemical treatment of human urine for its storage and reuse as flush water. Sci. Total Environ. 2007, 382, 159–164. [Google Scholar] [CrossRef]
- Mobley, H.; Hausinger, R. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Köninger, J.; Lugato, E.; Panagos, P.; Kochupillai, M.; Orgiazzi, A.; Briones, M.J. Manure management and soil biodiversity: Towards more sustainable food systems in the EU. Agric. Syst. 2021, 194, 103251. [Google Scholar] [CrossRef]
- Eurostat. Treatment of Waste by Waste Category, Hazardousness and Waste Management Operations for Animal Faeces, Manure and Urine; Eurostat: Luxembourg, 2021. [Google Scholar]
- European Commision. Implementation of Council Directive 91/676/EEC Concerning Theprotection of Waters against Pollution Caused by Nitrates from Agriculturalsources Based on Member State Reports for the Period 2016–2019; European Commision: Brussels, Belgium, 2021. [Google Scholar]
- Geng, Y.; Cao, G.; Wang, L.; Wang, S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef] [Green Version]
- Provolo, G.; Manuli, G.; Finzi, A.; Lucchini, G.; Riva, E.; Sacchi, G.A. Effect of pig and cattle slurry application on heavy metal composition of maize grown on different soils. Sustainability 2018, 10, 2684. [Google Scholar] [CrossRef] [Green Version]
- Kümmerer, K. Antibiotics in the aquatic environment—A review–part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Boyer, T.H.; Saetta, D. Opportunities for Building-Scale Urine Diversion and Challenges for Implementation. Acc. Chem. Res. 2019, 52, 886–895. [Google Scholar] [CrossRef]
- Simha, P. Alkaline Urine Dehydration. How to Dry Source Separated Human Urine and Recover Nutrients. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, May 2021. [Google Scholar]
- Lehtoranta, S.; Laukka, V.; Vidal, B.; Heiderscheidt, E.; Postila, H.; Nilivaara, R.; Herrmann, I. Circular Economy in Wastewater Management—The Potential of Source-Separating Sanitation in Rural and Peri-Urban Areas of Northern Finland and Sweden. Front. Environ. Sci. 2022, 10, 97. [Google Scholar] [CrossRef]
- Udert, K.; Larsen, T.A.; Gujer, W. Fate of major compounds in source-separated urine. Water Sci. Technol. 2006, 54, 413–420. [Google Scholar] [CrossRef]
- Larsen, T.A.; Alder, A.C.; Eggen, R.I.; Maurer, M.; Lienert, J. Source separation: Will we see a paradigm shift in wastewater handling? Environ. Sci. Technol. 2009, 43, 6121–6125. [Google Scholar] [CrossRef] [Green Version]
- Winblad, U. Small-scale systems for recycling of human excreta. Integrated Measures to Overcome Barriers to Minimizing Harmful Fluxes from Land to Water. In Proceedings of the 3rd Stockholm Water Symposium, Stockholm, Sweden, 10–14 August 1994; pp. 225–231. [Google Scholar]
- McConville, J.; Kvarnström, E.; Jönsson, H.; Kärrman, E.; Johansson, M. Source separation: Challenges & opportunities for transition in the swedish wastewater sector. Resour. Conserv. Recycl. 2017, 120, 144–156. [Google Scholar] [CrossRef]
- Oarga, A. Blackwater Treatment at Tourist Facilities. Ph.D. Thesis, University of Nova Gorica, Nova Gorica, Slovenia, 2013. [Google Scholar]
- United Nations. Stop Food Loss and Waste, for the People, for the Planet. Available online: https://www.un.org/en/observances/end-food-waste-day#:~:text=Globally%2C%20around%2014%20percent%20of,and%202%20percent%20in%20retail) (accessed on 20 November 2022).
- FAO. Food Wastage Footprint: Impacts on Natural Resources; FAO: Rome, Italy, 2013. [Google Scholar]
- Shi, X.; Zuo, J.; Li, B.; Yu, H. Two-stage anaerobic digestion of food waste coupled with in situ ammonia recovery using gas membrane absorption: Performance and microbial community. Bioresour. Technol. 2020, 297, 122458. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, Y.; Kato, H.; Li, Y.-Y. Enhancement of sustainable flux by optimizing filtration mode of a high-solid anaerobic membrane bioreactor during long-term continuous treatment of food waste. Water Res. 2020, 168, 115195. [Google Scholar] [CrossRef] [PubMed]
- Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Dry anaerobic digestion of chicken manure coupled with membrane separation of ammonia. Bioresour. Technol. 2017, 244, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Zhai, N.; Zhang, T.; Yin, D.; Yang, G.; Wang, X.; Ren, G.; Feng, Y. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manag. 2015, 38, 126–131. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2012. [Google Scholar]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Ahmad, A.; Mohd Said, N.S.; Mohd Rahim, N.F.; Mohammad Alnawajha, M.; Abu Hasan, H.; Othman, A.R.; Purwanti, I.F. Potential of valuable materials recovery from aquaculture wastewater: An introduction to resource reclamation. Aquac. Res. 2021, 52, 2954–2962. [Google Scholar] [CrossRef]
- Teoh, G.H.; Jawad, Z.A.; Ooi, B.S.; Low, S.C. Simultaneous water reclamation and nutrient recovery of aquaculture wastewater using membrane distillation. J. Water Process Eng. 2022, 46, 102573. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.F.; Mehrvar, M. Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: A review on trends and advances. J. Environ. Manag. 2015, 161, 287–302. [Google Scholar] [CrossRef]
- The World Bank. Water in Agriculture. Available online: https://www.worldbank.org/en/topic/water-in-agriculture (accessed on 4 November 2022).
- Jang, Y.; Lee, W.; Park, J.; Choi, Y. Recovery of ammonia from wastewater by liquid–liquid membrane contactor: A review. Membr. Water Treat. 2022, 13, 147–166. [Google Scholar] [CrossRef]
- Keller, J.; Yuan, Z. Integrating process engineering and microbiology tools to advance activated sludge wastewater treatment research and development. Rev. Environ. Sci. Biotechnol. 2002, 1, 83–97. [Google Scholar] [CrossRef]
- Qin, M.; Molitor, H.; Brazil, B.; Novak, J.T.; He, Z. Recovery of nitrogen and water from landfill leachate by a microbial electrolysis cell–forward osmosis system. Bioresour. Technol. 2016, 200, 485–492. [Google Scholar] [CrossRef]
- Righetto, I.; Al-Juboori, R.A.; Kaljunen, J.U.; Mikola, A. Multipurpose treatment of landfill leachate using natural coagulants–Pretreatment for nutrient recovery and removal of heavy metals and micropollutants. J. Environ. Chem. Eng. 2021, 9, 105213. [Google Scholar] [CrossRef]
- Di Iaconi, C.; Pagano, M.; Ramadori, R.; Lopez, A. Nitrogen recovery from a stabilized municipal landfill leachate. Bioresour. Technol. 2010, 101, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Kyllönen, H.; Heikkinen, J.; Järvelä, E.; Sorsamäki, L.; Siipola, V.; Grönroos, A. Wastewater purification with nutrient and carbon recovery in a mobile resource container. Membranes 2021, 11, 975. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Circular economy-driven ammonium recovery from municipal wastewater: State of the art, challenges and solutions forward. Bioresour. Technol. 2021, 334, 125231. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, Z.; Zhang, X.; Jin, Z.; Wang, C.; Zhang, L.; Wang, K. Organics and nitrogen recovery from sewage via membrane-based pre-concentration combined with ion exchange process. Chem. Eng. J. 2017, 311, 13–19. [Google Scholar] [CrossRef]
- Koskue, V.; Freguia, S.; Ledezma, P.; Kokko, M. Efficient nitrogen removal and recovery from real digested sewage sludge reject water through electroconcentration. J. Environ. Chem. Eng. 2021, 9, 106286. [Google Scholar] [CrossRef]
- Monetti, J.; Ledezma, P.; Virdis, B.; Freguia, S. Nutrient recovery by bio-electroconcentration is limited by wastewater conductivity. ACS Omega 2019, 4, 2152–2159. [Google Scholar] [CrossRef]
- Koskue, V.; Rinta-Kanto, J.M.; Freguia, S.; Ledezma, P.; Kokko, M. Optimising nitrogen recovery from reject water in a 3-chamber bioelectroconcentration cell. Sep. Purif. Technol. 2021, 264, 118428. [Google Scholar] [CrossRef]
- Ouma, J.; Septien, S.; Velkushanova, K.; Pocock, J.; Buckley, C. Characterization of ultrafiltration of undiluted and diluted stored urine. Water Sci. Technol. 2016, 74, 2105–2114. [Google Scholar] [CrossRef]
- Pocock, J.; Muzhingi, A.; Mercer, E.; Velkushnova, K.; Septien, S.; Buckley, C.A. Water and nutrient recovery from stored urine by forward osmosis with an ammonium bicarbonate draw solution. Front. Environ. Sci. 2022, 1239. [Google Scholar] [CrossRef]
- Tun, L.L.; Jeong, D.; Jeong, S.; Cho, K.; Lee, S.; Bae, H. Dewatering of source-separated human urine for nitrogen recovery by membrane distillation. J. Membr. Sci. 2016, 512, 13–20. [Google Scholar] [CrossRef]
- Monetti, J.; Ledezma, P.; Freguia, S. Optimised operational parameters for improved nutrient recovery from hydrolysed urine by bio-electroconcentration. Sep. Purif. Technol. 2021, 279, 119793. [Google Scholar] [CrossRef]
- Samanta, P.; Schönettin, H.M.; Horn, H.; Saravia, F. MF–NF Treatment Train for Pig Manure: Nutrient Recovery and Reuse of Product Water. Membranes 2022, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Jung, J.-Y.; Chung, Y.-C. Novel method for enhancing permeate flux of submerged membrane system in two-phase anaerobic reactor. Water Res. 2001, 35, 471–477. [Google Scholar] [CrossRef]
- Fugere, R.; Mameri, N.; Gallot, J.; Comeau, Y. Treatment of pig farm effluents by ultrafiltration. J. Membr. Sci. 2005, 255, 225–231. [Google Scholar] [CrossRef]
- López-Fernández, R.; Aristizábal, C.; Irusta, R. Ultrafiltration as an advanced tertiary treatment of anaerobically digested swine manure liquid fraction: A practical and theoretical study. J. Membr. Sci. 2011, 375, 268–275. [Google Scholar] [CrossRef]
- Masse, L.; Mondor, M.; Dubreuil, J. Membrane filtration of the liquid fraction from a solid–liquid separator for swine manure using a cationic polymer as flocculating agent. Environ. Technol. 2013, 34, 671–677. [Google Scholar] [CrossRef]
- Thörneby, L.; Persson, K.; Trägårdh, G. Treatment of liquid effluents from dairy cattle and pigs using reverse osmosis. J. Agric. Eng. Res. 1999, 73, 159–170. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.; Pellerin, Y.; Dubreuil, J. Osmotic pressure and substrate resistance during the concentration of manure nutrients by reverse osmosis membranes. J. Membr. Sci. 2010, 348, 28–33. [Google Scholar] [CrossRef]
- Mondor, M.; Masse, L.; Ippersiel, D.; Lamarche, F.; Masse, D. Use of electrodialysis and reverse osmosis for the recovery and concentration of ammonia from swine manure. Bioresour. Technol. 2008, 99, 7363–7368. [Google Scholar] [CrossRef]
- Carretier, S.; Lesage, G.; Grasmick, A.; Heran, M. Water and nutrients recovering from livestock manure by membrane processes. Can. J. Chem. Eng. 2015, 93, 225–233. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.; Pellerin, Y. The effect of pH on the separation of manure nutrients with reverse osmosis membranes. J. Membr. Sci. 2008, 325, 914–919. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Vanotti, M.B.; Hernández-González, D.; García-González, M.C. Pilot-scale demonstration of membrane-based nitrogen recovery from swine manure. Membranes 2020, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Schwark, L.v.U.-S.; Horn, H.; Saravia, F. Nutrient recovery and ammonia-water production by MF-vacuum evaporation treatment of pig manure. J. Environ. Chem. Eng. 2022, 10, 106929. [Google Scholar] [CrossRef]
- Besson, M.; Berger, S.; Tiruta-Barna, L.; Paul, E.; Spérandio, M. Environmental assessment of urine, black and grey water separation for resource recovery in a new district compared to centralized wastewater resources recovery plant. J. Clean. Prod. 2021, 301, 126868. [Google Scholar] [CrossRef]
- Van Voorthuizen, E.; Zwijnenburg, A.; van der Meer, W.; Temmink, H. Biological black water treatment combined with membrane separation. Water Res. 2008, 42, 4334–4340. [Google Scholar] [CrossRef] [PubMed]
- Hocaoglu, S.M.; Insel, G.; Cokgor, E.U.; Orhon, D. Effect of low dissolved oxygen on simultaneous nitrification and denitrification in a membrane bioreactor treating black water. Bioresour. Technol. 2011, 102, 4333–4340. [Google Scholar] [CrossRef]
- Hocaoglu, S.M.; Atasoy, E.; Baban, A.; Insel, G.; Orhon, D. Nitrogen removal performance of intermittently aerated membrane bioreactor treating black water. Environ. Technol. 2013, 34, 2717–2725. [Google Scholar] [CrossRef]
- Hafiza, N.; Abdillah, A.; Islami, B.; Priadi, C. Preliminary analysis of blackwater and greywater characteristics in the Jakarta Greater Region Area. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Xiamen, China, 7–9 June 2019; p. 012029. [Google Scholar]
- Shi, X.; Zuo, J.; Zhang, M.; Wang, Y.; Yu, H.; Li, B. Enhanced biogas production and in situ ammonia recovery from food waste using a gas-membrane absorption anaerobic reactor. Bioresour. Technol. 2019, 292, 121864. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Horváth, I.S.; Taherzadeh, M.J. Anaerobic digestion of food waste to volatile fatty acids and hydrogen at high organic loading rates in immersed membrane bioreactors. Renew. Energy 2020, 152, 1140–1148. [Google Scholar] [CrossRef]
- Zarrabi, M.; Mohammadi, A.A.; Al-Musawi, T.J.; Najafi Saleh, H. Using natural clinoptilolite zeolite as an amendment in vermicomposting of food waste. Environ. Sci. Pollut. Res. 2018, 25, 23045–23054. [Google Scholar] [CrossRef] [PubMed]
- Aji, N.A.S.; Yaser, A.; Lamaming, J.; Ugak, M.; Saalah, S.; Rajin, M. Production of food waste compost and its effect on the growth of dwarf crape jasmine. J. Kejuruter. 2021, 33, 413–424. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Lee, J.W.; Chandran, K.; Nhan, H.T.; Marcelino, K.R.; Khanal, S.K. Nitrogen recovery via aquaponics–bioponics: Engineering considerations and perspectives. ACS EST Eng. 2021, 1, 326–339. [Google Scholar] [CrossRef]
- Zou, S.; Guan, L.; Taylor, D.P.; Kuhn, D.; He, Z. Nitrogen removal from water of recirculating aquaculture system by a microbial fuel cell. Aquaculture 2018, 497, 74–81. [Google Scholar] [CrossRef]
- Mohammed, A.J.; Ismail, Z.Z. Slaughterhouse wastewater biotreatment associated with bioelectricity generation and nitrogen recovery in hybrid system of microbial fuel cell with aerobic and anoxic bioreactors. Ecol. Eng. 2018, 125, 119–130. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Treatment of poultry slaughterhouse wastewater using tubular microfiltration membrane with fly ash as key precursor. J. Water Process Eng. 2020, 37, 101361. [Google Scholar] [CrossRef]
- Brennan, B.; Gunes, B.; Jacobs, M.R.; Lawler, J.; Regan, F. Potential viable products identified from characterisation of agricultural slaughterhouse rendering wastewater. Water 2021, 13, 352. [Google Scholar] [CrossRef]
- Husam, A.-N.; Nassar, A. Slaughterhouses wastewater characteristics in the Gaza strip. J. Water Resour. Prot. 2019, 11, 844. [Google Scholar] [CrossRef] [Green Version]
- Kabdaşlı, I.; Tünay, O.; Özcan, P. Application of struvite precipitation coupled with biological treatment to slaughterhouse wastewaters. Environ. Technol. 2009, 30, 1095–1101. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Q. Recovery of ammonium-nitrogen from landfill leachate as a multi-nutrient fertilizer. Ecol. Eng. 2003, 20, 171–181. [Google Scholar] [CrossRef]
- Dos Santos, H.A.P.; de Castilhos Júnior, A.B.; Nadaleti, W.C.; Lourenço, V.A. Ammonia recovery from air stripping process applied to landfill leachate treatment. Environ. Sci. Pollut. Res. 2020, 27, 45108–45120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, W.; Zeng, X.; Xu, X. Recovery of ammonia nitrogen from landfill leachate using a biopolar membrane equipped electrodialysis system. Water Sci. Technol. 2020, 82, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Hybrid technologies: The future of energy efficient desalination—A review. Desalination 2020, 495, 114659. [Google Scholar] [CrossRef]
- Peters, T. Membrane technology for water treatment. Chem. Eng. Technol. 2010, 33, 1233–1240. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid–liquid separation of animal slurry in theory and practice. In Sustainable Agriculture Volume 2; Springer: Dordrecht, The Netherlands, 2011; pp. 953–986. [Google Scholar]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Selection of inorganic-based draw solutions for forward osmosis applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Singh, R.; Bhadouria, R.; Singh, P.; Kumar, A.; Pandey, S.; Singh, V.K. Nanofiltration technology for removal of pathogens present in drinking water. In Waterborne Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 463–489. [Google Scholar]
- Lee, S.; Lueptow, R.M. Membrane rejection of nitrogen compounds. Environ. Sci. Technol. 2001, 35, 3008–3018. [Google Scholar] [CrossRef]
- Ernst, M.; Bismarck, A.; Springer, J.; Jekel, M. Zeta-potential and rejection rates of a polyethersulfone nanofiltration membrane in single salt solutions. J. Membr. Sci. 2000, 165, 251–259. [Google Scholar] [CrossRef]
- Hoang, T.; Stevens, G.; Kentish, S. The effect of feed pH on the performance of a reverse osmosis membrane. Desalination 2010, 261, 99–103. [Google Scholar] [CrossRef]
- Zarebska, A.; Romero Nieto, D.; Christensen, K.V.; Fjerbæk Søtoft, L.; Norddahl, B. Ammonium fertilizers production from manure: A critical review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1469–1521. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Organic fouling and chemical cleaning of nanofiltration membranes: Measurements and mechanisms. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef]
- Hilal, N.; Ismail, A.F.; Wright, C. Membrane Fabrication; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Tawalbeh, M.; Al Mojjly, A.; Al-Othman, A.; Hilal, N. Membrane separation as a pre-treatment process for oily saline water. Desalination 2018, 447, 182–202. [Google Scholar] [CrossRef] [Green Version]
- Hilal, N.; Kochkodan, V. Surface modified microfiltration membranes with molecularly recognising properties. J. Membr. Sci. 2003, 213, 97–113. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Li, P.; Li, R.-H.; Li, X.-Y. Characterization and mitigation of the fouling of flat-sheet ceramic membranes for direct filtration of the coagulated domestic wastewater. J. Hazard. Mater. 2020, 385, 121557. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Meng, F.; Gong, H.; Wang, C.; Wang, K. Improved low-carbon-consuming fouling control in long-term membrane-based sewage pre-concentration: The role of enhanced coagulation process and air backflushing in sustainable sewage treatment. J. Membr. Sci. 2017, 529, 252–262. [Google Scholar] [CrossRef]
- Šostar-Turk, S.; Petrinić, I.; Simonič, M. Laundry wastewater treatment using coagulation and membrane filtration. Resour. Conserv. Recycl. 2005, 44, 185–196. [Google Scholar] [CrossRef]
- Ravazzini, A.; Van Nieuwenhuijzen, A.; Van Der Graaf, J. Direct ultrafiltration of municipal wastewater: Comparison between filtration of raw sewage and primary clarifier effluent. Desalination 2005, 178, 51–62. [Google Scholar] [CrossRef]
- Cosenza, A.; Gulhan, H.; Maida, C.M.; Mannina, G. Nutrient recovery from wastewater treatment by ultrafiltration membrane for water reuse in view of a circular economy perspective. Bioresour. Technol. 2022, 363, 127929. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Aljohani, N.H.; Oatley-Radcliffe, D.L.; Lovitt, R.W. Moving towards sustainable resources: Recovery and fractionation of nutrients from dairy manure digestate using membranes. Water Res. 2015, 80, 80–89. [Google Scholar] [CrossRef]
- Pronk, W.; Palmquist, H.; Biebow, M.; Boller, M. Nanofiltration for the separation of pharmaceuticals from nutrients in source-separated urine. Water Res. 2006, 40, 1405–1412. [Google Scholar] [CrossRef]
- Halim, N.S.A.; Jusoh, A.; Endut, A. The formation and characterisation of an asymmetric nanofiltration membrane for ammonia–nitrogen removal: Effect of shear rate. Bioresour. Technol. 2010, 101, 1459–1465. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, X.; Chen, Y. Fit-for-purpose design of nanofiltration membranes for simultaneous nutrient recovery and micropollutant removal. Environ. Sci. Technol. 2021, 55, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Bilstad, T.; Madland, M.; Espedal, E.; Hanssen, P. Membrane separation of raw and anaerobically digested pig manure. Water Sci. Technol. 1992, 25, 19–26. [Google Scholar] [CrossRef]
- Ledda, C.; Schievano, A.; Salati, S.; Adani, F. Nitrogen and water recovery from animal slurries by a new integrated ultrafiltration, reverse osmosis and cold stripping process: A case study. Water Res. 2013, 47, 6157–6166. [Google Scholar] [CrossRef] [PubMed]
- Mavhungu, A.; Masindi, V.; Foteinis, S.; Mbaya, R.; Tekere, M.; Kortidis, I.; Chatzisymeon, E. Advocating circular economy in wastewater treatment: Struvite formation and drinking water reclamation from real municipal effluents. J. Environ. Chem. Eng. 2020, 8, 103957. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Lu, K.J.; Chen, Y.; Chung, T.-S. Design of omniphobic interfaces for membrane distillation—A review. Water Res. 2019, 162, 64–77. [Google Scholar] [CrossRef]
- Qu, D.; Sun, D.; Wang, H.; Yun, Y. Experimental study of ammonia removal from water by modified direct contact membrane distillation. Desalination 2013, 326, 135–140. [Google Scholar] [CrossRef]
- Wu, C.; Yan, H.; Li, Z.; Lu, X. Ammonia recovery from high concentration wastewater of soda ash industry with membrane distillation process. Desalin. Water Treat. 2016, 57, 6792–6800. [Google Scholar] [CrossRef]
- Xie, Z.; Duong, T.; Hoang, M.; Nguyen, C.; Bolto, B. Ammonia removal by sweep gas membrane distillation. Water Res. 2009, 43, 1693–1699. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Xia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct contact membrane distillation for the treatment of industrial dyeing wastewater and characteristic pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R.; Hilal, N. Alternative heating techniques in membrane distillation: A review. Desalination 2020, 496, 114713. [Google Scholar] [CrossRef]
- Usman, H.S.; Touati, K.; Rahaman, M.S. An economic evaluation of renewable energy-powered membrane distillation for desalination of brackish water. Renew. Energy 2021, 169, 1294–1304. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; Li, Z.; Ma, R.; Yang, Z. Experimental study of ammonia removal from water by membrane distillation (MD): The comparison of three configurations. J. Membr. Sci. 2006, 286, 93–103. [Google Scholar] [CrossRef]
- Davey, C.J.; Liu, P.; Kamranvand, F.; Williams, L.; Jiang, Y.; Parker, A.; Tyrrel, S.; McAdam, E.J. Membrane distillation for concentrated blackwater: Influence of configuration (air gap, direct contact, vacuum) on selectivity and water productivity. Sep. Purif. Technol. 2021, 263, 118390. [Google Scholar] [CrossRef]
- Khan, A.; Yadav, S.; Ibrar, I.; Al Juboori, R.A.; Razzak, S.A.; Deka, P.; Subbiah, S.; Shah, S. Fouling and Performance Investigation of Membrane Distillation at Elevated Recoveries for Seawater Desalination and Wastewater Reclamation. Membranes 2022, 12, 951. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, K.; Yu, H.; Liang, H.; Xie, B.; Li, G.; Qu, F.; van der Bruggen, B. Treatment of anaerobic digestion effluent using membrane distillation: Effects of feed acidification on pollutant removal, nutrient concentration and membrane fouling. Desalination 2019, 449, 6–15. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, M.; Chen, G.; Huang, M.; Tan, W. Nitrogen recovery from a palladium leachate via membrane distillation: System performance and ammonium chloride crystallization. Resour. Conserv. Recycl. 2022, 183, 106368. [Google Scholar] [CrossRef]
- Zico, M.; Ricci, B.; Reis, B.; Magalhães, N.; Amaral, M. Sustainable ammonia resource recovery from landfill leachate by solar-driven modified direct contact membrane distillation. Sep. Purif. Technol. 2021, 264, 118356. [Google Scholar] [CrossRef]
- Huo, X.; Vanneste, J.; Cath, T.Y.; Strathmann, T.J. A hybrid catalytic hydrogenation/membrane distillation process for nitrogen resource recovery from nitrate-contaminated waste ion exchange brine. Water Res. 2020, 175, 115688. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yang, G.; Zhang, J.; Xie, Z. De-ammonification using direct contact membrane distillation—An experimental and simulation study. Sep. Purif. Technol. 2020, 250, 117158. [Google Scholar] [CrossRef]
- He, Q.; Tu, T.; Yan, S.; Yang, X.; Duke, M.; Zhang, Y.; Zhao, S. Relating water vapor transfer to ammonia recovery from biogas slurry by vacuum membrane distillation. Sep. Purif. Technol. 2018, 191, 182–191. [Google Scholar] [CrossRef]
- Yang, X.; Fraser, T.; Myat, D.; Smart, S.; Zhang, J.; Diniz da Costa, J.C.; Liubinas, A.; Duke, M. A pervaporation study of ammonia solutions using molecular sieve silica membranes. Membranes 2014, 4, 40–54. [Google Scholar] [CrossRef] [Green Version]
- El-Bourawi, M.; Khayet, M.; Ma, R.; Ding, Z.; Li, Z.; Zhang, X. Application of vacuum membrane distillation for ammonia removal. J. Membr. Sci. 2007, 301, 200–209. [Google Scholar] [CrossRef]
- Qiu, B.; Fan, S.; Tang, X.; Qi, B.; Deng, L.; Wang, W.; Liu, J.; Wang, Y.; Xiao, Z. Simultaneous recovery of phosphorus and nitrogen from liquid digestate by vacuum membrane distillation with permeate fractional condensation. Chin. J. Chem. Eng 2020, 28, 1558–1565. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Zhu, J. Study on treatment of wastewater with low concentration of ammonia-nitrogen by vacuum plate membrane distillation technology. Water Sci. Technol. 2022, 86, 950–967. [Google Scholar] [CrossRef]
- Shi, M.; Xiao, M.; Feng, L.; Tu, T.; He, Q.; Yan, S. Water and green ammonia recovery from anaerobic digestion effluent by two-stage membrane distillation. J. Water Process Eng. 2022, 49, 102949. [Google Scholar] [CrossRef]
- Zhao, Z.-P.; Xu, L.; Shang, X.; Chen, K. Water regeneration from human urine by vacuum membrane distillation and analysis of membrane fouling characteristics. Sep. Purif. Technol. 2013, 118, 369–376. [Google Scholar] [CrossRef]
- Shi, M.; He, Q.; Feng, L.; Wu, L.; Yan, S. Techno-economic evaluation of ammonia recovery from biogas slurry by vacuum membrane distillation without pH adjustment. J. Clean. Prod. 2020, 265, 121806. [Google Scholar] [CrossRef]
- Tibi, F.; Guo, J.; Ahmad, R.; Lim, M.; Kim, M.; Kim, J. Membrane distillation as post-treatment for anaerobic fluidized bed membrane bioreactor for organic and nitrogen removal. Chemosphere 2019, 234, 756–762. [Google Scholar] [CrossRef]
- Jacob, P.; Phungsai, P.; Fukushi, K.; Visvanathan, C. Direct contact membrane distillation for anaerobic effluent treatment. J. Membr. Sci. 2015, 475, 330–339. [Google Scholar] [CrossRef]
- Jafarinejad, S. Forward osmosis membrane technology for nutrient removal/recovery from wastewater: Recent advances, proposed designs, and future directions. Chemosphere 2021, 263, 128116. [Google Scholar] [CrossRef] [PubMed]
- Haupt, A.; Lerch, A. Forward osmosis application in manufacturing industries: A short review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Gray, G.T.; McCutcheon, J.R.; Elimelech, M. Internal concentration polarization in forward osmosis: Role of membrane orientation. Desalination 2006, 197, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.; Tang, C.Y. Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation: Advances, challenges, and prospects for the future. J. Membr. Sci. 2016, 504, 113–132. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. High performance thin-film composite forward osmosis membrane. Environ. Sci. Technol. 2010, 44, 3812–3818. [Google Scholar] [CrossRef]
- Loeb, S.; Titelman, L.; Korngold, E.; Freiman, J. Effect of porous support fabric on osmosis through a Loeb-Sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- Phuntsho, S.; Lotfi, F.; Hong, S.; Shaffer, D.L.; Elimelech, M.; Shon, H.K. Membrane scaling and flux decline during fertiliser-drawn forward osmosis desalination of brackish groundwater. Water Res. 2014, 57, 172–182. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Wang, Y.-N.; Wang, R.; Tang, C.Y. Comparison of NF-like and RO-like thin film composite osmotically-driven membranes—Implications for membrane selection and process optimization. J. Membr. Sci. 2013, 427, 460–471. [Google Scholar] [CrossRef]
- She, Q.; Jin, X.; Li, Q.; Tang, C.Y. Relating reverse and forward solute diffusion to membrane fouling in osmotically driven membrane processes. Water Res. 2012, 46, 2478–2486. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Yuan, B.; Li, X.; Ren, Y. Impacts of sludge retention time on sludge characteristics and membrane fouling in a submerged osmotic membrane bioreactor. Bioresour. Technol. 2014, 161, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Lay, W.C.; Zhang, Q.; Zhang, J.; McDougald, D.; Tang, C.; Wang, R.; Liu, Y.; Fane, A.G. Effect of pharmaceuticals on the performance of a novel osmotic membrane bioreactor (OMBR). Sep. Sci. Technol. 2012, 47, 543–554. [Google Scholar] [CrossRef]
- Yogalakshmi, K.; Joseph, K. Effect of transient sodium chloride shock loads on the performance of submerged membrane bioreactor. Bioresour. Technol. 2010, 101, 7054–7061. [Google Scholar] [CrossRef]
- Qiu, G.; Ting, Y.-P. Short-term fouling propensity and flux behavior in an osmotic membrane bioreactor for wastewater treatment. Desalination 2014, 332, 91–99. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Yusaf, T. Biofouling in RO system: Mechanisms, monitoring and controlling. Desalination 2012, 302, 1–23. [Google Scholar] [CrossRef]
- Tang, C.Y.; She, Q.; Lay, W.C.; Wang, R.; Fane, A.G. Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J. Membr. Sci. 2010, 354, 123–133. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Gao, S.; Zhang, Z.; Cui, F.; Tang, C.Y. In situ surface modification of thin film composite forward osmosis membranes with sulfonated poly (arylene ether sulfone) for anti-fouling in emulsified oil/water separation. J. Membr. Sci. 2017, 527, 26–34. [Google Scholar] [CrossRef]
- Xue, W.; Yamamoto, K.; Tobino, T. Membrane fouling and long-term performance of seawater-driven forward osmosis for enrichment of nutrients in treated municipal wastewater. J. Membr. Sci. 2016, 499, 555–562. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; He, T.; Price, W.E.; Nghiem, L.D. Physical cleaning techniques to control fouling during the pre-concentration of high suspended-solid content solutions for resource recovery by forward osmosis. Desalination 2018, 429, 134–141. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Boo, C.; Elimelech, M.; Hong, S. Fouling control in a forward osmosis process integrating seawater desalination and wastewater reclamation. J. Membr. Sci. 2013, 444, 148–156. [Google Scholar] [CrossRef]
- Bell, E.A.; Poynor, T.E.; Newhart, K.B.; Regnery, J.; Coday, B.D.; Cath, T.Y. Produced water treatment using forward osmosis membranes: Evaluation of extended-time performance and fouling. J. Membr. Sci. 2017, 525, 77–88. [Google Scholar] [CrossRef]
- Seman, M.A.; Khayet, M.; Hilal, N. Development of antifouling properties and performance of nanofiltration membranes modified by interfacial polymerisation. Desalination 2011, 273, 36–47. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.; Matsuura, T.; Hilal, N.; Ismail, A. The potential of thin film nanocomposite membrane in reducing organic fouling in forward osmosis process. Desalination 2014, 348, 82–88. [Google Scholar] [CrossRef]
- Hilal, N.; Khayet, M.; Wright, C.J. Membrane Modification: Technology and Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Xu, W.; Chen, Q.; Ge, Q. Recent advances in forward osmosis (FO) membrane: Chemical modifications on membranes for FO processes. Desalination 2017, 419, 101–116. [Google Scholar] [CrossRef]
- Giwa, A.; Hasan, S.; Yousuf, A.; Chakraborty, S.; Johnson, D.; Hilal, N. Biomimetic membranes: A critical review of recent progress. Desalination 2017, 420, 403–424. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Kim, Y.; Chekli, L.; Phuntsho, S.; Shon, H.K.; Leiknes, T.; Ghaffour, N. Impact of reverse nutrient diffusion on membrane biofouling in fertilizer-drawn forward osmosis. J. Membr. Sci. 2017, 539, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Yamamoto, K.; Nghiem, L.D. High retention membrane bioreactors: Challenges and opportunities. Bioresour. Technol. 2014, 167, 539–546. [Google Scholar] [CrossRef]
- Yao, X.; Gonzales, R.R.; Sasaki, Y.; Lin, Y.; Shen, Q.; Zhang, P.; Shintani, T.; Nakagawa, K.; Matsuyama, H. Surface modification of FO membrane for improving ammoniacal nitrogen (NH4+-N) rejection: Investigating the factors influencing NH4+-N rejection. J. Membr. Sci. 2022, 650, 120429. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Xie, M.; Zhang, B.; Li, G.; Luo, W. Resource recovery from digested manure centrate: Comparison between conventional and aquaporin thin-film composite forward osmosis membranes. J. Membr. Sci. 2020, 593, 117436. [Google Scholar] [CrossRef]
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s potential: An overview of draw solutes for forward osmosis. Desalination 2018, 434, 100–120. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Toward resource recovery from wastewater: Extraction of phosphorus from digested sludge using a hybrid forward osmosis–membrane distillation process. Environ. Sci. Technol. Lett. 2014, 1, 191–195. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- She, Q.; Wong, Y.K.W.; Zhao, S.; Tang, C.Y. Organic fouling in pressure retarded osmosis: Experiments, mechanisms and implications. J. Membr. Sci. 2013, 428, 181–189. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Hai, F.I.; Ansari, A.J.; Roddick, F.A. Mining phosphorus from anaerobically treated dairy manure by forward osmosis membrane. J. Ind. Eng. Chem. 2019, 78, 425–432. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Selection of forward osmosis draw solutes for subsequent integration with anaerobic treatment to facilitate resource recovery from wastewater. Bioresour. Technol. 2015, 191, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Phosphorus recovery from digested sludge centrate using seawater-driven forward osmosis. Sep. Purif. Technol. 2016, 163, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Fang, Z.; Liang, P.; Huang, X. Direct concentration of municipal sewage by forward osmosis and membrane fouling behavior. Bioresour. Technol. 2018, 247, 730–735. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Chen, S.-S.; Yang, H.-Y.; Hau, N.T. Application of forward osmosis on dewatering of high nutrient sludge. Bioresour. Technol. 2013, 132, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; She, Q.; Chang, V.W.C.; Tang, C.Y.; Webster, R.D. Mining nutrients (N, K, P) from urban source-separated urine by forward osmosis dewatering. Environ. Sci. Technol. 2014, 48, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Devia, Y.P.; Imai, T.; Higuchi, T.; Kanno, A.; Yamamoto, K.; Sekine, M.; Van Le, T. Potential of magnesium chloride for nutrient rejection in forward osmosis. J. Water Resour. Prot. 2015, 7, 730. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zheng, J.; Tang, J.; Wang, X.; Wu, Z. A pilot-scale forward osmosis membrane system for concentrating low-strength municipal wastewater: Performance and implications. Sci. Rep. 2016, 6, 21653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.-Z.; Wang, L.-F.; Pan, X.-R.; Zhang, F.; Huang, M.-S.; Li, W.-W.; Liu, H.-Q. Selective separation of volatile fatty acids, nitrogen and phosphorus from anaerobic acidogenic fermentation via forward osmosis membrane process. Chem. Eng. J. 2022, 453, 139871. [Google Scholar] [CrossRef]
- Kong, F.-X.; Dong, L.-Q.; Zhang, T.; Chen, J.-F.; Guo, C.-M. Effect of reverse permeation of draw solute on the rejection of ionic nitrogen inorganics in forward osmosis: Comparison, prediction and implications. Desalination 2018, 437, 144–153. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, S.; Zhang, B.; Wang, L.; He, Z. Forward osmosis promoted in-situ formation of struvite with simultaneous water recovery from digested swine wastewater. Chem. Eng. J. 2018, 342, 274–280. [Google Scholar] [CrossRef]
- Abdul Wahid, R.; Ang, W.L.; Mohammad, A.W.; Johnson, D.J.; Hilal, N. Evaluating fertilizer-drawn forward osmosis performance in treating anaerobic palm oil mill effluent. Membranes 2021, 11, 566. [Google Scholar] [CrossRef]
- Hafiz, M.A.; Hawari, A.H.; Altaee, A. A hybrid forward osmosis/reverse osmosis process for the supply of fertilizing solution from treated wastewater. J. Water Process Eng. 2019, 32, 100975. [Google Scholar] [CrossRef]
- Rood, B.J. Forward Osmosis with an Algal Draw Solution for Wastewater Concentrating and Polishing. Ph.D. Thesis, University of Missouri-Columbia, Columbia, MO, USA, May 2018. [Google Scholar]
- Jafarinejad, S.; Park, H.; Mayton, H.; Walker, S.L.; Jiang, S.C. Concentrating ammonium in wastewater by forward osmosis using a surface modified nanofiltration membrane. Environ. Sci. Water Res. Technol. 2019, 5, 246–255. [Google Scholar] [CrossRef]
- Camilleri-Rumbau, M.S.; Soler-Cabezas, J.L.; Christensen, K.V.; Norddahl, B.; Mendoza-Roca, J.A.; Vincent-Vela, M.C. Application of aquaporin-based forward osmosis membranes for processing of digestate liquid fractions. Chem. Eng. J. 2019, 371, 583–592. [Google Scholar] [CrossRef]
- Schneider, C.; Rajmohan, R.S.; Zarebska, A.; Tsapekos, P.; Hélix-Nielsen, C. Treating anaerobic effluents using forward osmosis for combined water purification and biogas production. Sci. Total Environ. 2019, 647, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dhiman, S.; Basu, S.; Balakrishnan, M.; Petrinic, I.; Helix-Nielsen, C. Dewatering of sewage for nutrients and water recovery by Forward Osmosis (FO) using divalent draw solution. J. Water Process Eng. 2019, 31, 100853. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Sasaki, Y.; Istirokhatun, T.; Li, J.; Matsuyama, H. Ammonium enrichment and recovery from synthetic and real industrial wastewater by amine-modified thin film composite forward osmosis membranes. Sep. Purif. Technol. 2022, 297, 121534. [Google Scholar] [CrossRef]
- Englande, A., Jr.; Krenkel, P.; Shamas, J. Wastewater treatment &water reclamation. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Judd, S.J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2016, 305, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Jain, M. Anaerobic membrane bioreactor as highly efficient and reliable technology for wastewater treatment—A review. Adv. Chem. Eng. Sci. 2018, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer–can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H.; Lee, D.-J.; Tung, K.-L.; Jin, P.; Wang, J.; Wu, Y. Challenges in biogas production from anaerobic membrane bioreactors. Renew. Energy 2016, 98, 120–134. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Pan, S.-Y.; Lin, Y.-I.; Cao, T.N.-D. Anaerobic swine digestate valorization via energy-efficient electrodialysis for nutrient recovery and water reclamation. Water Res. 2022, 224, 119066. [Google Scholar] [CrossRef]
- González, E.; Díaz, O.; Ruigómez, I.; de Vera, C.; Rodríguez-Gómez, L.; Rodríguez-Sevilla, J.; Vera, L. Photosynthetic bacteria-based membrane bioreactor as post-treatment of an anaerobic membrane bioreactor effluent. Bioresour. Technol. 2017, 239, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, W.; Van de Caveye, P.; Diamantis, V. Maximum use of resources present in domestic “used water”. Bioresour. Technol. 2009, 100, 5537–5545. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, J.; Plaza, F.; Garralon, G.; Fdz-Polanco, F.; Peña, M. Long-term operation of a pilot scale anaerobic membrane bioreactor (AnMBR) for the treatment of municipal wastewater under psychrophilic conditions. Bioresour. Technol. 2015, 185, 225–233. [Google Scholar] [CrossRef]
- Sierra, J.D.M.; Oosterkamp, M.J.; Wang, W.; Spanjers, H.; van Lier, J.B. Impact of long-term salinity exposure in anaerobic membrane bioreactors treating phenolic wastewater: Performance robustness and endured microbial community. Water Res. 2018, 141, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Siles, J.; Brekelmans, J.; Martín, M.; Chica, A.; Martín, A. Impact of ammonia and sulphate concentration on thermophilic anaerobic digestion. Bioresour. Technol. 2010, 101, 9040–9048. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Pidou, M.; Soares, A.; Judd, S.; Jefferson, B. Modelling the energy demands of aerobic and anaerobic membrane bioreactors for wastewater treatment. Environ. Technol. 2011, 32, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Skouteris, G.; Hermosilla, D.; López, P.; Negro, C.; Blanco, Á. Anaerobic membrane bioreactors for wastewater treatment: A review. Chem. Eng. J. 2012, 198, 138–148. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Cao, C.; Zhang, J.; Ng, J.-W.; Tang, C. Performance of a submerged anaerobic membrane bioreactor with forward osmosis membrane for low-strength wastewater treatment. Water Res. 2014, 50, 114–123. [Google Scholar] [CrossRef]
- Gao, Y.; Haavisto, S.; Li, W.; Tang, C.Y.; Salmela, J.; Fane, A.G. Novel approach to characterizing the growth of a fouling layer during membrane filtration via optical coherence tomography. Environ. Sci. Technol. 2014, 48, 14273–14281. [Google Scholar] [CrossRef]
- Alturki, A.; McDonald, J.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Performance of a novel osmotic membrane bioreactor (OMBR) system: Flux stability and removal of trace organics. Bioresour. Technol. 2012, 113, 201–206. [Google Scholar] [CrossRef]
- Qiu, G.; Ting, Y.-P. Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Bioresour. Technol. 2014, 170, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, B.; Chen, Y.; Li, X.; Ren, Y. Integration of micro-filtration into osmotic membrane bioreactors to prevent salinity build-up. Bioresour. Technol. 2014, 167, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Law, Y.-M.; Das, S.; Ting, Y.-P. Direct and complete phosphorus recovery from municipal wastewater using a hybrid microfiltration-forward osmosis membrane bioreactor process with seawater brine as draw solution. Environ. Sci. Technol. 2015, 49, 6156–6163. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wu, Y.; Lu, Y.X.; Cai, Y.; He, Z.; Yang, X.L.; Song, H.L. A comprehensive review of nutrient-energy-water-solute recovery by hybrid osmotic membrane bioreactors. Bioresour. Technol. 2021, 320, 124300. [Google Scholar] [CrossRef]
- Chang, H.-M.; Sun, Y.-C.; Chien, I.-C.; Chang, W.-S.; Ray, S.S.; Cao, D.T.N.; Duong, C.C.; Chen, S.-S. Innovative upflow anaerobic sludge osmotic membrane bioreactor for wastewater treatment. Bioresour. Technol. 2019, 287, 121466. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, L.; Ng, J.-W.; Lee, C.; Chang, V.W.-C.; Tang, C.Y. Development of anaerobic osmotic membrane bioreactor for low-strength wastewater treatment at mesophilic condition. J. Membr. Sci. 2015, 490, 197–208. [Google Scholar] [CrossRef]
- Qiu, G.; Ting, Y.P. Osmotic membrane bioreactor for wastewater treatment and the effect of salt accumulation on system performance and microbial community dynamics. Bioresour. Technol. 2013, 150, 287–297. [Google Scholar] [CrossRef]
- Holloway, R.W.; Wait, A.S.; da Silva, A.F.; Herron, J.; Schutter, M.D.; Lampi, K.; Cath, T.Y. Long-term pilot scale investigation of novel hybrid ultrafiltration-osmotic membrane bioreactors. Desalination 2015, 363, 64–74. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Yuan, B.; Wang, Z.; Li, X.; Ren, Y. Comparison of biofouling mechanisms between cellulose triacetate (CTA) and thin-film composite (TFC) polyamide forward osmosis membranes in osmotic membrane bioreactors. Bioresour. Technol. 2016, 202, 50–58. [Google Scholar] [CrossRef]
- Linares, R.V.; Li, Z.; Abu-Ghdaib, M.; Wei, C.-H.; Amy, G.; Vrouwenvelder, J.S. Water harvesting from municipal wastewater via osmotic gradient: An evaluation of process performance. J. Membr. Sci. 2013, 447, 50–56. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Abouhend, A.S.; McNair, A.; Kuo-Dahab, W.C.; Watt, C.; Butler, C.S.; Milferstedt, K.; Hamelin, J.; Seo, J.; Gikonyo, G.J.; El-Moselhy, K.M. The oxygenic photogranule process for aeration-free wastewater treatment. Environ. Sci. Technol. 2018, 52, 3503–3511. [Google Scholar] [CrossRef]
- Acién, F.G.; Gómez-Serrano, C.; Morales-Amaral, M.D.M.; Fernández-Sevilla, J.M.; Molina-Grima, E. Wastewater treatment using microalgae: How realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Ding, A.; Bui, X.T.; Nguyen, D.P. Bio-membrane based integrated systems for nitrogen recovery in wastewater treatment: Current applications and future perspectives. Chemosphere 2021, 265, 129076. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Monfet, E.; Unc, A. Defining wastewaters used for cultivation of algae. Algal Res. 2017, 24, 520–526. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, Z.; Mei, J.; Liang, Z.; Li, Z.; Hou, X. Investigation into the novel microalgae membrane bioreactor with internal circulating fluidized bed for marine aquaculture wastewater treatment. Membranes 2020, 10, 353. [Google Scholar] [CrossRef]
- Goh, P.S.; Ahmad, N.A.; Lim, J.W.; Liang, Y.Y.; Kang, H.S.; Ismail, A.F.; Arthanareeswaran, G. Microalgae-Enabled Wastewater Remediation and Nutrient Recovery through Membrane Photobioreactors: Recent Achievements and Future Perspective. Membranes 2022, 12, 1094. [Google Scholar] [CrossRef]
- Lee, D.-J.; Hsieh, M.-H. Forward osmosis membrane processes for wastewater bioremediation: Research needs. Bioresour. Technol. 2019, 290, 121795. [Google Scholar] [CrossRef]
- Xu, M.; Li, P.; Tang, T.; Hu, Z. Roles of SRT and HRT of an algal membrane bioreactor system with a tanks-in-series configuration for secondary wastewater effluent polishing. Ecol. Eng. 2015, 85, 257–264. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Jiménez-Benítez, A.; Pachés, M.; Ruano, M.V.; Borrás, L.; Barat, R.; Seco, A. Improving membrane photobioreactor performance by reducing light path: Operating conditions and key performance indicators. Water Res. 2020, 172, 115518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, E.; Babaei, A.; Mehrnia, M.R.; Shayegan, J.; Safdari, M.-S. Municipal wastewater treatment by semi-continuous and membrane algal-bacterial photo-bioreactors. J. Water Process Eng. 2020, 36, 101274. [Google Scholar] [CrossRef]
- Praveen, P.; Heng, J.Y.P.; Loh, K.-C. Tertiary wastewater treatment in membrane photobioreactor using microalgae: Comparison of forward osmosis & microfiltration. Bioresour. Technol. 2016, 222, 448–457. [Google Scholar] [CrossRef]
- Zou, H.; Rutta, N.C.; Chen, S.; Zhang, M.; Lin, H.; Liao, B. Membrane Photobioreactor Applied for Municipal Wastewater Treatment at a High Solids Retention Time: Effects of Microalgae Decay on Treatment Performance and Biomass Properties. Membranes 2022, 12, 564. [Google Scholar] [CrossRef]
- Urtiaga, A. Electrochemical technologies combined with membrane filtration. Curr. Opin. Electrochem. 2021, 27, 100691. [Google Scholar] [CrossRef]
- Swanckaert, B.; Geltmeyer, J.; Rabaey, K.; De Buysser, K.; Bonin, L.; De Clerck, K. A review on ion-exchange nanofiber membranes: Properties, structure and application in electrochemical (waste) water treatment. Sep. Purif. Technol. 2022, 287, 120529. [Google Scholar] [CrossRef]
- Mondal, R.; Pal, S.; Patnaik, P.; Bhalani, D.V.; Gupta, S.; Chatterjee, U.; Jewrajka, S.K. Influence of the formed interface during preparation of poly (vinylidene fluoride) blend cation exchange membrane on the electro-chemical properties and performance. Desalination 2022, 531, 115682. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Ion-exchange membrane systems—Electrodialysis and other electromembrane processes. In Fundamental Modelling of Membrane Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 251–300. [Google Scholar]
- Biesheuvel, P.; Van der Wal, A. Membrane capacitive deionization. J. Membr. Sci. 2010, 346, 256–262. [Google Scholar] [CrossRef]
- Sun, J.; Xu, W.; Cai, B.; Huang, G.; Zhang, H.; Zhang, Y.; Yuan, Y.; Chang, K.; Chen, K.; Peng, Y. High-concentration nitrogen removal coupling with bioelectric power generation by a self-sustaining algal-bacterial biocathode photo-bioelectrochemical system under daily light/dark cycle. Chemosphere 2019, 222, 797–809. [Google Scholar] [CrossRef]
- Wu, X.; Modin, O. Ammonium recovery from reject water combined with hydrogen production in a bioelectrochemical reactor. Bioresour. Technol. 2013, 146, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.Y.; Kaksonen, A.H.; Cord-Ruwisch, R. Ammonia recycling enables sustainable operation of bioelectrochemical systems. Bioresour. Technol. 2013, 143, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangadharan, P.; Vadekeetil, A.; Sibi, R.; Sheelam, A. Concentrating nutrients and recovering water and energy from source separated urine using osmotic microbial fuel cell. Chemosphere 2021, 285, 131548. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, H.; Tan, G.; Wen, F.; Flynn, M.T.; Zhu, X. Resource recovery microbial fuel cells for urine-containing wastewater treatment without external energy consumption. Chem. Eng. J. 2019, 373, 1072–1080. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Santoro, C.; Serov, A.; Atanassov, P.; Melhuish, C.; Ieropoulos, I.A. Multi-functional microbial fuel cells for power, treatment and electro-osmotic purification of urine. J. Chem. Technol. Biotechnol. 2019, 94, 2098–2106. [Google Scholar] [CrossRef] [Green Version]
- Jiaqi, S.; Lifen, L.; Fenglin, Y. Successful bio-electrochemical treatment of nitrogenous mariculture wastewater by enhancing nitrogen removal via synergy of algae and cathodic photo-electro-catalysis. Sci. Total Environ. 2020, 743, 140738. [Google Scholar] [CrossRef]
- Wang, C.; Sun, M.; Wang, X.; Zhang, X.; Zhao, Y.; Huo, M. Enhanced resource recovery from wastewater using electrochemical-osmotic system with nanofiltration membranes. Resour. Conserv. Recycl. 2022, 186, 106555. [Google Scholar] [CrossRef]
- Koskue, V.; Monetti, J.; Rossi, N.; Nieradzik, L.; Freguia, S.; Kokko, M.; Ledezma, P. Fate of pharmaceuticals and PFASs during the electrochemical generation of a nitrogen-rich nutrient product from real reject water. J. Environ. Chem. Eng. 2022, 10, 107284. [Google Scholar] [CrossRef]
- Sakar, H.; Celik, I.; Balcik-Canbolat, C.; Keskinler, B.; Karagunduz, A. Ammonium removal and recovery from real digestate wastewater by a modified operational method of membrane capacitive deionization unit. J. Clean. Prod. 2019, 215, 1415–1423. [Google Scholar] [CrossRef]
- Li, G.; Cao, Y.; Zhang, Z.; Hao, L. Removal of ammonia nitrogen from water by mesoporous carbon electrode–based membrane capacitance deionization. Environ. Sci. Pollut. Res. 2021, 28, 7945–7954. [Google Scholar] [CrossRef]
- Baghodrat, M.; Mehri, F.; Rowshanzamir, S. Electrochemical performance and enhanced nitrate removal of homogeneous polysulfone-based anion exchange membrane applied in membrane capacitive deionization cell. Desalination 2020, 496, 114696. [Google Scholar] [CrossRef]
- Chen, L.; He, F.; Li, F. Denitrification enhancement by electro-adsorption/reduction in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) with copper electrode. Chemosphere 2022, 291, 132732. [Google Scholar] [CrossRef] [PubMed]
- Tarpeh, W.A.; Barazesh, J.M.; Cath, T.Y.; Nelson, K.L. Electrochemical Stripping to Recover Nitrogen from Source-Separated Urine. Environ. Sci. Technol. 2018, 52, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Molenaar, S.; Barbosa, J.; Sleutels, T.; Hamelers, H.V.; Buisman, C.J.; Kuntke, P. Effluent pH correlates with electrochemical nitrogen recovery efficiency at pilot scale operation. Sep. Purif. Technol. 2022, 306, 122602. [Google Scholar] [CrossRef]
- Zamora, P.; Georgieva, T.; Ter Heijne, A.; Sleutels, T.H.; Jeremiasse, A.W.; Saakes, M.; Buisman, C.J.; Kuntke, P. Ammonia recovery from urine in a scaled-up microbial electrolysis cell. J. Power Source 2017, 356, 491–499. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, K.; He, C.; Wang, K. Ammonia removal from municipal wastewater via membrane capacitive deionization (MCDI) in pilot-scale. Sep. Purif. Technol. 2022, 286, 120469. [Google Scholar] [CrossRef]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow fibre membrane contactors for ammonia recovery: Current status and future developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Boehler, M.A.; Heisele, A.; Seyfried, A.; Grömping, M.; Siegrist, H. (NH4)2SO4 recovery from liquid side streams. Environ. Sci. Pollut. Res. 2015, 22, 7295–7305. [Google Scholar] [CrossRef]
- Davey, C.J.; Hermassi, M.; Allard, E.; Amine, M.; Sweet, N.; Gaite, T.S.; McLeod, A.; McAdam, E.J. Integrating crystallisation into transmembrane chemical absorption: Process intensification for ammonia separation from anaerobic digestate. J. Membr. Sci. 2020, 611, 118236. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Naji, O.; Bowtell, L.; Alpatova, A.; Soukane, S.; Ghaffour, N. Power effect of ultrasonically vibrated spacers in air gap membrane distillation: Theoretical and experimental investigations. Sep. Purif. Technol. 2021, 262, 118319. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Romero, J.; Pérez, B.; Plaza, A. Ammonia removal from wastewater streams through membrane contactors: Experimental and theoretical analysis of operation parameters and configuration. Chem. Eng. J. 2010, 160, 530–537. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.; Khorasani, Z. Ammonia removal from aqueous solutions using hollow-fiber membrane contactors. Chem. Eng. J. 2010, 162, 242–249. [Google Scholar] [CrossRef]
- Tan, X.; Tan, S.; Teo, W.K.; Li, K. Polyvinylidene fluoride (PVDF) hollow fibre membranes for ammonia removal from water. J. Membr. Sci. 2006, 271, 59–68. [Google Scholar] [CrossRef]
- Ahn, Y.; Hwang, Y.-H.; Shin, H.-S. Application of PTFE membrane for ammonia removal in a membrane contactor. Water Sci. Technol. 2011, 63, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Righetto, I.; Al-Juboori, R.A.; Kaljunen, J.U.; Mikola, A. Wastewater treatment with starch-based coagulants for nutrient recovery purposes: Testing on lab and pilot scales. J. Environ. Manag. 2021, 284, 112021. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, Y.; Wang, F.; Zhang, H.; Guo, Y. Long-term stability of polytetrafluoroethylene (PTFE) hollow fiber membranes for CO2 capture. Energy Fuels 2016, 30, 492–503. [Google Scholar] [CrossRef]
- Brennan, B.; Lawler, J.; Regan, F. Recovery of viable ammonia–nitrogen products from agricultural slaughterhouse wastewater by membrane contactors: A review. Environ. Sci. Water Res. Technol. 2021, 7, 259–273. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Rostami, E.; Rahimpour, A. Surfactant cleaning of ultrafiltration membranes fouled by whey. Int. J. Dairy Technol. 2010, 63, 273–283. [Google Scholar] [CrossRef]
- Rudolph, G.; Schagerlöf, H.; Morkeberg Krogh, K.B.; Jönsson, A.-S.; Lipnizki, F. Investigations of alkaline and enzymatic membrane cleaning of ultrafiltration membranes fouled by thermomechanical pulping process water. Membranes 2018, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Tijing, L.D.; Woo, Y.C.; Johir, M.A.H.; Choi, J.-S.; Shon, H.K. A novel dual-layer bicomponent electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Chem. Eng. J. 2014, 256, 155–159. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dube, P.; Vanotti, M.; Szogi, A.; García-González, M. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Application of gas-permeable membranes for-semi-continuous ammonia recovery from swine manure. Environments 2019, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Fillingham, M.; VanderZaag, A.C.; Singh, J.; Burtt, S.; Crolla, A.; Kinsley, C.; MacDonald, J.D. Characterizing the performance of gas-permeable membranes as an ammonia recovery strategy from anaerobically digested dairy manure. Membranes 2017, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xie, M.; Tong, X.; Yang, D.; Liu, S.; Qu, D.; Feng, L.; Zhang, L. Ammonia capture from human urine to harvest liquid NP compound fertilizer by a submerged hollow fiber membrane contactor: Performance and fertilizer analysis. Sci. Total Environ. 2021, 768, 144478. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, M.; Yang, D.; Tong, X.; Qu, D.; Feng, L.; Zhang, L. The design of multi-stage open-loop hollow fiber membrane contactor and its application in ammonia capture from hydrolyzed human urine. Water Res. 2021, 207, 117811. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, J.; Xie, M.; Tong, X.; Jiang, T.; Yu, W.; Qu, D. An integrated hollow fiber membrane contactor and chemical precipitation to recover N, P and K from human urine wastewater. J. Environ. Chem. Eng. 2022, 10, 107844. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Mikola, A.; Heinonen-Tanski, H.; Vahala, R. Recovery of nitrogen and phosphorus from human urine using membrane and precipitation process. J. Environ. Manag. 2019, 247, 596–602. [Google Scholar] [CrossRef]
- González-Camejo, J.; Montero, P.; Aparicio, S.; Ruano, M.V.; Borrás, L.; Seco, A.; Barat, R. Nitrite inhibition of microalgae induced by the competition between microalgae and nitrifying bacteria. Water Res. 2020, 172, 115499. [Google Scholar] [CrossRef]
- Munasinghe-Arachchige, S.P.; Nirmalakhandan, N. Nitrogen-fertilizer recovery from the centrate of anaerobically digested sludge. Environ. Sci. Technol. Lett. 2020, 7, 450–459. [Google Scholar] [CrossRef]
- Pretel, R.; Durán, F.; Robles, A.; Ruano, M.; Ribes, J.; Serralta, J.; Ferrer, J. Designing an AnMBR-based WWTP for energy recovery from urban wastewater: The role of primary settling and anaerobic digestion. Sep. Purif. Technol. 2015, 156, 132–139. [Google Scholar] [CrossRef]
- Prieto, A.L.; Futselaar, H.; Lens, P.N.L.; Bair, R.; Yeh, D.H. Development and start up of a gas-lift anaerobic membrane bioreactor (Gl-AnMBR) for conversion of sewage to energy, water and nutrients. J. Membr. Sci. 2013, 441, 158–167. [Google Scholar] [CrossRef]
- McCartney, S.N.; Williams, N.A.; Boo, C.; Chen, X.; Yip, N.Y. Novel Isothermal Membrane Distillation with Acidic Collector for Selective and Energy-Efficient Recovery of Ammonia from Urine. ACS Sustain. Chem. Eng. 2020, 8, 7324–7334. [Google Scholar] [CrossRef]
- Arredondo, M.R.; Kuntke, P.; Ter Heijne, A.; Hamelers, H.V.; Buisman, C.J. Load ratio determines the ammonia recovery and energy input of an electrochemical system. Water Res. 2017, 111, 330–337. [Google Scholar] [CrossRef]
- Jiang, H.; Straub, A.P.; Karanikola, V. Ammonia Recovery with Sweeping Gas Membrane Distillation: Energy and Removal Efficiency Analysis. ACS EST Eng. 2022, 2, 617–628. [Google Scholar] [CrossRef]
- Sayegh, A.; Prakash, N.S.; Horn, H.; Saravia, F. Membrane distillation as a second stage treatment of hydrothermal liquefaction wastewater after ultrafiltration. Sep. Purif. Technol. 2022, 285, 120379. [Google Scholar] [CrossRef]
- Qin, M.; White, C.; Zou, S.; He, Z. Passive separation of recovered ammonia from catholyte for reduced energy consumption in microbial electrolysis cells. Chem. Eng. J. 2018, 334, 2303–2307. [Google Scholar] [CrossRef]
- Kuntke, P.; Śmiech, K.; Bruning, H.; Zeeman, G.; Saakes, M.; Sleutels, T.; Hamelers, H.; Buisman, C. Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 2012, 46, 2627–2636. [Google Scholar] [CrossRef]
- Wäeger-Baumann, F.; Fuchs, W. The application of membrane contactors for the removal of ammonium from anaerobic digester effluent. Sep. Sci. Technol. 2012, 47, 1436–1442. [Google Scholar] [CrossRef]
- Vanotti, M.; Dube, P.; Szogi, A.; García-González, M. Recovery of ammonia and phosphate minerals from swine wastewater using gas-permeable membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntke, P.; Arredondo, M.R.; Widyakristi, L.; Ter Heijne, A.; Sleutels, T.H.; Hamelers, H.V.; Buisman, C.J. Hydrogen gas recycling for energy efficient ammonia recovery in electrochemical systems. Environ. Sci. Technol. 2017, 51, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Kuntke, P.; Zamora, P.; Saakes, M.; Buisman, C.; Hamelers, H. Gas-permeable hydrophobic tubular membranes for ammonia recovery in bio-electrochemical systems. Environ. Sci. Water Res. Technol. 2016, 2, 261–265. [Google Scholar] [CrossRef]
- Tice, R.C.; Kim, Y. Energy efficient reconcentration of diluted human urine using ion exchange membranes in bioelectrochemical systems. Water Res. 2014, 64, 61–72. [Google Scholar] [CrossRef]
- Hou, D.; Lu, L.; Sun, D.; Ge, Z.; Huang, X.; Cath, T.Y.; Ren, Z.J. Microbial electrochemical nutrient recovery in anaerobic osmotic membrane bioreactors. Water Res. 2017, 114, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.; Paradkar, A.; Sleutels, T.; Ter Heijne, A.; Buisman, C.J.; Hamelers, H.V.; Kuntke, P. Donnan Dialysis for scaling mitigation during electrochemical ammonium recovery from complex wastewater. Water Res. 2021, 201, 117260. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, L.; Wang, Y.; Shehzad, M.A.; Xu, T. Ammonia capture by water splitting and hollow fiber extraction. Chem. Eng. Sci. 2018, 192, 211–217. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; He, D.; Waite, T.D. Capacitive Membrane Stripping for Ammonia Recovery (CapAmm) from Dilute Wastewaters. Environ. Sci. Techno. Lett. 2018, 5, 43–49. [Google Scholar] [CrossRef]
- Guo, X.; Chen, J.; Wang, X.; Li, Y.; Liu, Y.; Jiang, B. Sustainable ammonia recovery from low strength wastewater by the integrated ion exchange and bipolar membrane electrodialysis with membrane contactor system. Sep. Purif. Technol. 2022, 305, 122429. [Google Scholar] [CrossRef]
- Hou, D.; Lu, L.; Ren, Z.J. Microbial fuel cells and osmotic membrane bioreactors have mutual benefits for wastewater treatment and energy production. Water Res. 2016, 98, 183–189. [Google Scholar] [CrossRef]
- Yang, Y.-L.; He, Z.; Wu, Y.; Yang, X.-L.; Cai, Y.; Song, H.-L. Bioelectrochemically assisted osmotic membrane bioreactor with reusable polyelectrolyte draw solutes. Bioresour. Technol. 2020, 296, 122352. [Google Scholar] [CrossRef] [PubMed]
- Zgavarogea, R.; Covaliu, C.; Iordache, A.; Niculescu, V.; Neacsa, M. The use of anaerobic membrane bioreactor and reverse osmosis system for wastewater treatment. Univ. Politeh. Buchar. Sci. Bull. Ser. C Electr. Eng. Comput. Sci. 2017, 79, 55–62. [Google Scholar]
- Zoungrana, A.; Zengin, İ.H.; Türk, O.K.; Çakmakcı, M. Ammoniacal nitrogen reclamation by membrane distillation from high ammonia polluted solutions. Chem. Pap. 2020, 74, 1903–1915. [Google Scholar] [CrossRef]















| Originally Captured Keywords | Replacement |
|---|---|
| Ammonia capture | Nitrogen recovery |
| Ammonia recovery | |
| Ammonia removal | |
| Ammonium recovery | |
| Ammonium removal | |
| nitrogen recovery | |
| nitrogen removal | |
| nutrient recovery | |
| nutrient removal | |
| nutrients recovery | |
| nutrients removal | |
| microbial fuel cell | microbial fuel cell |
| microbial fuel-cell | |
| microbial fuel-cells | |
| reverse osmosis | Reverse Osmosis |
| reverse-osmosis | |
| bioreactor | Bioreactor |
| bioreactors | |
| fuel-cell | Fuel-cell |
| fuel-cells | |
| bioreactor | Bioreactor |
| bioreactors | |
| gas-permeable membrane | Gas-permeable membrane |
| gas-permeable membranes | |
| MBR | Membrane bioreactor |
| membrane bioreactor | |
| membrane contactor | Membrane contactor |
| membrane contactors | |
| membrane technology | Membrane technology |
| membranes | |
| municipal waste-water | Wastewater |
| municipal wastewater | |
| waste-water | |
| wastewater | |
| wastewaters | |
| sewage treatment | Sewage treatment |
| sewage-treatment |
| Waste Stream | N Concentration (g/L) | Solids Content (g/L) | Other Characteristics | Ref. | ||
|---|---|---|---|---|---|---|
| pH | COD (g/L) | Conductivity (mS/cm) | ||||
| Domestic Wastewater | 0.025–1.2 | 0.2–0.8 | 6.5–8, | 0.17–0.9 | 1.2–18 | [17,27,29,37,40,77,78,79,80,81,82] |
| Urine | 0.2–8.5 | 0.25–0.32 | 6–11 | 1–20 | 26–50 | [42,43,83,84,85,86] |
| Manure slurry | 1–5.5 | 0.5–15 | 7–9 | 5–45 | 1–24 | [19,87,88,89,90,91,92,93,94,95,96,97,98] |
| Source separated black water | 0.12–1.2 | 0.2–1.6 | 7–9 | 0.5–8 | 1.9–8 | [60,99,100,101,102,103] |
| Food waste * | 4–3 | 13–45 | 4–5.5 | 73–160 ** | 7.5–9.5 | [63,64,104,105,106,107] |
| Aquaculture wastewater | 0.0003–0.016 | 0.001–0.08 | 6–8 | 0.008–0.14 | 0.8–2.3 | [68,69,108,109] |
| Slaughterhouse wastewater | 0.030–0.2 | 0.2–0.45 | 6–7.5 | 0.25–11.5 | 1–4 | [110,111,112,113,114] |
| Landfill leachate | 1–4.5 | 0.025–9 | 7.8–8.5 | 1.5–10 | 2.5–28 | [10,74,76,115,116,117] |
| Membrane Technology | Membrane Material | Feed Type | Flux (LMH) | NH4+ Rejection (%) | Ref. |
|---|---|---|---|---|---|
| MF | Aluminum oxide (Al2O3) ceramic membranes | Filtered sow slurry (15–20 g/L) | - | Slurry volume reduced by 70% | [19] |
| α-Al2O3 flat sheet ceramic membranes | Pre-coagulated domestic wastewater | 41.7 | 40–50 | [132] | |
| PVDF | Pre-coagulated raw sewage | - | 28–52 * | [133] | |
| PVDF | Raw sewage | 5–10 | 37.5 | [79] | |
| UF | PVDF | Pig manure after settling tank | ~9 * | Concentration factor of 3.7 | [89] |
| Pig manure after screening | ~7 * | Concentration factor of 3.7 | |||
| Pig manure after screening + settling | ~10 * | Concentration factor of 2.2 | |||
| Pig manure after screening + settling + aerobic bioreactor | ~34 * | Concentration factor of 4.3 | |||
| Multi-channel membrane with an active surface layers made of Al2O3, TiO2 and ZrO2 | Laundry wastewater | ~130 * | ~99 | [134] | |
| PVDF | Primary clarifier effluent | 91–168 depending on flow velocity and applied pressure | Only 10% rejection of total N but nor rejection of NH4+ | [135] | |
| PVDF | screen raw sewage with 0.56 mm sieve | 70–110 depending on flow velocity and applied pressure | Only 10% rejection of total N but nor rejection of NH4+ | ||
| PVDF | Activated sludge effluent | ~14 * | 0–58% rejection when varying filtration/backwash time ration of 5–9 | [136] | |
| NF | PA active layer+ PSU support layer | Dairy manure digestate | 125–150 at pH = 11 | ~32 | [137] |
| PA active layer+ PSU support layer | Synthetic urine | ~130–170 for pH = 3–9 | 45 * | [138] | |
| PES | Aquaculture effluent | ~8–18 for 2–10 bars | 68 | [139] | |
| PA | Synthetic wastewater with micropollutants | - | 60 | [140] | |
| RO | PA | Anaerobically digested pig manure | ~10–68 * depending on the concentration factor achieved | 95 | [141] |
| PA active layer+ PSU support layer | Prefiltered Sow slurry with MF | - | ~91 * | [19] | |
| PA | Prefiltered heifer wastewater by 30 μm filter | 30 | 96 | [92] | |
| PA | Pre-treated swine manure with diatomaceous earth | - | ~98 for pH 4.5–7 | [96] | |
| PA | Pre-filtered swine manure | - | 66.6 | [94] | |
| PA | Pre-filtered swine manure | ~30 * | Concentration factor of 5.6 | [93] | |
| RO (Dow, USA) | Pre-treated digested cattle manure with screw press separation + centrifugation + UF | - | 99.5 | [142] | |
| Pre-treated digested swine manure with screw press separation + centrifugation + UF | - | 96 | |||
| PA | Effluent of fluidized bed reactor + anaerobic membrane bioreactor | 12.3 | 94–100 for pH = 8–4 | [29] | |
| PA | Municipal wastewater | ~52 * | ~100 | [143] |
| Configuration | Membrane Material | Feed Type | NH4+ Rejection (%) | Ref. |
|---|---|---|---|---|
| DCMD | PTFE | palladium leachate | 97.4 | [158] |
| Modified DCMD by solar energy system | PP | landfill leachate | 59 | [159] |
| DCMD with acid absorption | PVDF | Synthetic NH4Cl solution | 99.5 | [146] |
| DCMD with acid absorption | PTFE | Ion exchange brine | >96 | [160] |
| DCMD | Nafion ionomer and Multiwall Carbon Nanotubes (MWCNTs)+ a Poly (vinylidene fluoride-cohexafluoropropene; PVDF-HFP) | Sludge digestate | ~5–60 for pH = 7–12 | [24] |
| DCMD | PTFE with PP scrim backing | Synthetic ammonia solution | 90 | [161] |
| VMD | PP | Biogas slurry | 98 | [162] |
| VMD | PP | Synthetic solution of NH4OH | Concentration factor of ~10–15 | [163] |
| VMD | PTFE | Synthetic solution of NH4OH | 90 | [164] |
| VMD | PTFE | Liquid digestate | ~95 | [165] |
| VMD | PTFE | Simulated wastewater made of NH4Cl, Na2CO3 and Na2SO4 | 93.3 at pH = 4 | [166] |
| Two stages DCMD | PP | Anaerobic digestion effluent | ~81 | [167] |
| VMD | PTFE | Human urine | 40–75 | [168] |
| VMD | PTFE | Biogas slurry | Concentration factor of 8 | [169] |
| SGMD | PTFE | Synthetic ammonia solution | 97 | [148] |
| Membrane bioreactor (MBR) + DCMD | PVDF | Synthetic NH4Cl solution | 76–94 | [170] |
| MBR + DCMD | PTFE | Anaerobic effluent | 89.6–96.3 | [171] |
| Membrane Materials & Orientation | Waste Stream | Draw Solution | Operational Mode | NH4+ Rejection (%) | Flux | Ref. |
|---|---|---|---|---|---|---|
| Flat sheet CTA, asymmetric | Spiked activated sludge with glucose, NH4Cl and K2HPO4 | NaCl | FO | 96 | 2.5–6.15 | [211] |
| Flat sheet CTA, symmetric | Synthetic hydrolyzed urine | NaCl | FO | 50–80 | 10–24 | [212] |
| Flat sheet CTA with nonwoven support layer, asymmetric | Synthetic secondary treated wastewater effluent | MgCl2 | FO | 99.4 | ~10 | [213] |
| Spiral-wound CTA | Real domestic wastewater | NaCl | FO | 48 | 6 | [214] |
| Flat sheet CTA embedded in polyester mesh support, asymmetric | MBR effluent | Synthetic sea water | FO | Concentrated by 2.1-fold | ~4.8–5.5 | [189] |
| Flat sheet CTA embedded in polyester mesh support | Anaerobic acidogenic fermentation of anaerobic MBR | NaCl | FO | 92–97 for pH = 3–7 | ~14 | [215] |
| Flat sheet CTA embedded in polyester mesh support | Real municipal wastewater | NaCl | FO | 93 | Initial flux varied: ~8–25 with draw solution concentration change of 0.5–4 M | [210] |
| Flat sheet CTA embedded in polyester mesh support | NH4Cl dissolved in background electrolyte solution (10 mmol/L NaCl + 0.1 mmol/L NaHCO3) | NaCl | FO | 4.5–78 for water flux of ~3.5–18 LMH | ~3.5–18 for NaCl concentration of 0.25–3.0 mol/L | [216] |
| Flat sheet CTA | Centrate of digested swine wastewater | MgCl2 | FO | NH4+ penetration was desirable rather than rejection (93% of NH4+ passed through) | Maximum of 3.1 | [217] |
| Flat sheet CTA | Anaerobically treated palm oil mill effluent | (NH4)2SO4 | FO | Concertation factor of 0.7 | ~2.1 | [218] |
| NH4H2PO4 | Concertation factor of 1.65 | ~2.6 | ||||
| KCl | Concertation factor of 1.8 | ~1.9 | ||||
| Flat sheet polyamide TFC | NH4Cl dissolved in background electrolyte solution (10 mmol/L NaCl + 0.1 mmol/L NaHCO3) | NaCl | FO | <10 for all tested water fluxes | ~11–32 for NaCl concentration of 0.25–3.0 mol/L | [216] |
| Flat sheet TFC | Treated sewage effluent | NaCl + (NH4)2HPO4 | FO | 97 | ~13 | [219] |
| Flat sheet TFC | Treated sewage effluent | NaCl + (NH4)2HPO4 | PRO | 95 | ~10.5 | [219] |
| Flat sheet TFC, symmetric | Synthetic municipal wastewater | Synthetic seawater | FO | 67 | 12 | [220] |
| Virgin polyamide (PA) TFC | Synthetic ammonium solutions | MgCl2 | FO | ~97 | 0.7 | [221] |
| Virgin polyamide (PA) TFC | Secondary return activated sludge | MgCl2 | FO | 75.5 | ~0.6 | [221] |
| Surface modified PA TFC (grafted with 3% polyethylenimine (PEI)) | Synthetic ammonium solutions | MgCl2 | FO | 100 | ~1.3 | [221] |
| Surface modified PA TFC (grafted with 1.5% polyethylenimine (PEI)) | Secondary return activated sludge | MgCl2 | FO | ~89 | ~0.3 | [221] |
| Aquaporin Inside™ TFC flat sheet | Centrate of cow manure digestion | NaCl | FO | ~95 | ~7.5 | [222] |
| Aquaporin Inside™ TFC flat sheet | Centrate of cow manure digestion | Hide preservation wastewater | FO | ~95 | ~6.3 | [222] |
| Aquaporin Inside™ TFC flat sheet | Anaerobic digester effluent | MgCl2 | FO | ~97 | ~2–3.3 | [223] |
| Aquaporin Inside™ TFC flat sheet | Sewage | MgCl2 | FO | 66 | 5.3 | [224] |
| Aquaporin A/S TFC flat sheet | Centrate of digested swine farm | NaCl | FO | ~40 | Water flux varied between ~6 and ~4 for water recovery of 10% and 50%, respectively | [202] |
| Flat sheet TFC | Centrate of digested swine farm | NaCl | FO | ~45 | Water flux varied between 6 and ~1.5 for water recovery of 10% and 50%, respectively | [202] |
| PA flat sheet TFC | NH4Cl | NaCl | FO | ~40 | ~14 | [225] |
| PA flat sheet TFC grafted with quaternized polyethyleneimine | NH4Cl | NaCl | FO | ~95 | ~6.5 | [225] |
| OMBR Configuration | Waste Stream | Draw Solution | NH4+-N Rejection (%) | Ref. |
|---|---|---|---|---|
| Submerged aerobic OMBR (plate and frame) | Synthetic domestic wastewater | NaCl | 90 | [248] |
| Submerged aerobic OMBR (plate and frame) | Synthetic domestic wastewater | NaCl | >60 | [240] |
| Submerged aerobic OMBR (FO cell) | Synthetic wastewater | NaCl | 70–80 | [249] |
| Submerged aerobic OMBR (plate and frame) | Activated sludge | NaCl | 97 | [243,250] |
| Submerged aerobic OMBR (plate and frame) with UF membrane | Activated sludge | NaCl | >80 | [251] |
| Side-stream OMBR (plate and frame) | Activated sludge | NaCl | 98 | [252] |
| Submerged anoxic OMBR (plate and frame) | Synthetic wastewater | NaCl | 68 | [253] |
| Anaerobic submerged OMBR tubular module with MF membrane and moving sponge | Real domestic wastewater | A mixture of Na3PO4 and EDTA | 75 | [246] |
| Anaerobic submerged OMBR tubular module with UASB | Anaerobic granular sludge | MgSO4 | 55–86 | [247] |
| Process | Wastewater Stream | Nitrogen Recovery | Energy Consumption | Ref. |
|---|---|---|---|---|
| bipolar electrodialysis (pilot plan) | source-separated diluted urine | 88% of the NH4+ | 13 Wh/gN | [287] |
| microbial fuel cell (bio-electrochemical system) | mariculture wastewater | 94.05% NH4+-N and 77.35% inorganic nitrogen | - | [279] |
| combination of electrodialysis and membrane stripping | source-separated urine | 93% ammonium sulfate | 30.6 MJ kg N−1 | [286] |
| carbon electrode–based membrane capacitance deionization | Simulated wastewater | 82.33% NH4+, 90.96% NO2−, and 97.73% NO3− | - | [283] |
| membrane capacitive deionization (pilot-scale) | Municipal wastewater | 39.12 ± 5.31% Ammonia | 1.16 kWh/m3 | [289] |
| enhanced Flowed Channel Membrane Capacitive Deionization | digestate wastewater | 89% and 67% with synthetic and real digestate wastewater+ | - | [282] |
| Membrane Type | Waste Stream | pH Raising Agent | Stripping Solution | NH4+-N Recovery (%) | Scale | Ref. |
|---|---|---|---|---|---|---|
| Expanded polytetrafluoroethylene (ePTFE) | Diluted swine manure | Aeration+NaHCO3+N-Allylthiourea (nitrification inhibitor) | H2SO4 | 99 | Lab | [31] |
| ePTFE | The effluent of anaerobically digested swine wastewater | Aeration+nitrapyrin (nitrification inhibitor) | H2SO4 | 96–98 | Lab | [306] |
| ePTFE | Swine manure centrate | Aeration+Allylthiourea | H2SO4 | 90 | Lab | [307] |
| ePTFE | Raw manure and digestate of dairy farm | - | H2SO4 | ~3–13 | Lab | [308] |
| PP | Centrate of anaerobic digester of domestic wastewater treatment | NaOH | H2SO4 | >99 | Lab | [25] |
| ePTFE | Treated mesophilic digester wastewater with ballasted sedimentation | Slaked lime | H2SO4 | 55 | Pilot | [26] |
| HNO3 | 41 | |||||
| H3PO4 | 48 | |||||
| ePTFE | Treated mesophilic digester wastewater with ballasted | Slaked lime | H2SO4 | 92.5 | Pilot | [23] |
| Landfill leachate | NaOH | 86.5 | ||||
| Human urine | Slaked lime | 70 | ||||
| PP | Synthetic hydrolyzed human urine | KOH | H3PO4 | 90.9 | Lab | [309] |
| PP | Hydrolyzed human urine | NaOH | H2SO4 | 93 | Lab | [310] |
| PP | Synthetic hydrolyzed human urine | NaOH | H3PO4 | 97 | Lab | [311] |
| PTFE | Hydrolyzed urine | Ca(OH)2 | H2SO4 | 98 | Lab | [312] |
| System | Waste Stream | Energy Requirements | NH4+-N Recovery/Concentration | Scale | Ref. | |
|---|---|---|---|---|---|---|
| RO | Animal manure | 4.3–5.5 kWh/m3 feed | 90–98% retention | Pilot | [127] | |
| MD | VMD | Biogas slurry | 9.5–32.6 kWh/m3 feed * | 87% | Lab | [169] |
| DCMD | Anaerobic digester effluent | 8.6–45.4 kWh/m3 feed * | >98% | Lab | [167] | |
| SGMD | Simulated centrate of domestic wastewater digested | 1.42 kWh/m3 feed * | 99% | Modeling | [319] | |
| AGMD | Filtered hydrothermal liquefaction wastewater with UF | 16.6 kWh/m3 feed ◊ | Obtained 80% water recovery with an NH4+ concentration of 13 g/L | Lab | [320] | |
| AnMBR | Domestic wastewater | 0.03–5.7 | - | Modeling | [238] | |
| ECMs | MEC | Synthetic wastewater and simulated anaerobic digestion effluent | 1.3–4.0 kWh/kgN | 90–94% | Lab | [274,321] |
| MFC | Urine | 1.86 kWh/kgN | 0.32g N/d.m2 | Lab | [322] | |
| GPM | Digested and centrifuged animal manure, and influent of anaerobic digester | 0.17–1.2kWh/kgN § | 90–98% | Lab | [306,307,308,323,324] | |
| Landfill leachate and urine | 8.8–11.4 kWh/m3 feed | 70–86.5 | Pilot | [23] | ||
| Hydrogen recycling electrochemical system (HRES) + GPM | Urine | 8.5 kWh/kgN with a current intensity of 10 A/m2 | 64 | Lab | [325] | |
| 7.3 kWh/kgN with a current intensity of 20 A/m2 | 73 | |||||
| 15.7 kWh/kgN with a current intensity of 50 A/m2 | 60 | |||||
| MEC + GPM | Urine | 5.6–13.8 kWh/kgN | 90 | Lab | [318] | |
| MEC + GPM | Urine | 1.4 kWh/kgN | 31 | Pilot | [288] | |
| Urine | 2.5 kWh/kgN | 49 | Lab | [326] | ||
| MEC + Ion exchange membrane | Urine | 1.8 kWh/kgN | Ammonia concentrated by a factor of 4.5 | Lab | [327] | |
| MEC + AnOMBR | Synthetic wastewater | 40 kWh/kgN § | 45 | Lab | [328] | |
| MEC + GPM | Synthetic influent black water | 9.7 kWh/kgN | 83 | Lab | [329] | |
| Bipolar MEC + GPM | Synthetic wastewater | 0.76 kWh/kgN | 65 | Lab | [330] | |
| Capacitive deionization membrane+ GPM | Synthetic wastewater | 9.9–21.1 kWh/kgN | 60 | Lab | [331] | |
| MEC + GPM | Synthetic wastewater | 8.12–11.9 kWh/kgN | 9–74 | Lab | [332] | |
| MFC + OMBR | Synthetic wastewater | 1.23 kWh/m3 feed # | Concentrated NH4+ from 20 to 30 mg/L at the start and then went back to 20 mg/L | Lab | [333] | |
| MEC + OMBR | Synthetic wastewater | 1.23 kWh/m3 feed # | 72–78.5 | Lab | [334] | |
| RO + AnMBR | Domestic wastewater | 3–6 kWh/m3 feed | >90% concentrated N | Pilot | [20] | |
| RO + AnMBR | Domestic wastewater | 3–7 kWh/m3 feed | >90% concentrated N | Pilot | [335] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Juboori, R.A.; Al-Shaeli, M.; Aani, S.A.; Johnson, D.; Hilal, N. Membrane Technologies for Nitrogen Recovery from Waste Streams: Scientometrics and Technical Analysis. Membranes 2023, 13, 15. https://doi.org/10.3390/membranes13010015
Al-Juboori RA, Al-Shaeli M, Aani SA, Johnson D, Hilal N. Membrane Technologies for Nitrogen Recovery from Waste Streams: Scientometrics and Technical Analysis. Membranes. 2023; 13(1):15. https://doi.org/10.3390/membranes13010015
Chicago/Turabian StyleAl-Juboori, Raed A., Muayad Al-Shaeli, Saif Al Aani, Daniel Johnson, and Nidal Hilal. 2023. "Membrane Technologies for Nitrogen Recovery from Waste Streams: Scientometrics and Technical Analysis" Membranes 13, no. 1: 15. https://doi.org/10.3390/membranes13010015
APA StyleAl-Juboori, R. A., Al-Shaeli, M., Aani, S. A., Johnson, D., & Hilal, N. (2023). Membrane Technologies for Nitrogen Recovery from Waste Streams: Scientometrics and Technical Analysis. Membranes, 13(1), 15. https://doi.org/10.3390/membranes13010015