Double-Layered Pebax® 3533/ZIF-8 Membranes with Single-Walled Carbon Nanotube Buckypapers as Support for Gas Separation
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Single-Walled Carbon Nanotube Buckypaper (SWCNT-bp) Preparation
2.3. ZIF-8 Synthesis on SWCNT-bp
2.4. Preparation of Pebax® 3533 Dense Membranes
2.5. Preparation of Pebax® 3533 Supported Membranes by Phase Inversion (PI)
2.6. Preparation of Pebax® 3533 Supported Membranes by Spin Coating (SC)
2.7. Membrane Characterization
2.8. Mixed Gas Permeation
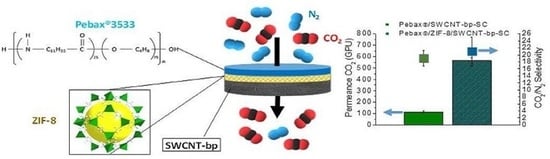
3. Results and Discussion
3.1. Membrane Characterization
3.2. Mixture Gas Permeation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Solomon, S.; Plattner, G.K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Martínez-Izquierdo, L.; Malankowska, M.; Sánchez-Laínez, J.; Téllez, C.; Coronas, J. Poly (ether-block-amide) copolymer membrane for CO2/N2 separation: The influence of the casting solution concentration on its morphology, thermal properties and gas separation performance. R. Soc. Open Sci. 2022, 6, 190866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazani, F.; Aroujalian, A. Enhanced CO2-selective behavior of Pebax-1657: A comparative study between the influence of graphene-based fillers. Polym. Test. 2020, 81, 106264. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Knoef, M.H.; Nijmeijer, K.; Wessling, M. Poly (ethylene glycol) and poly (dimethyl siloxane): Combining their advantages into efficient CO2 gas separation membranes. J. Memb. Sci. 2010, 352, 126–135. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Benito, J.; Sánchez-Laínez, J.; Zornoza, B.; Martín, S.; Carta, M.; Malpass-Evans, R.; Téllez, C.; McKeown, N.B.; Coronas, J.; Gascón, I. Ultrathin Composite Polymeric Membranes for CO2/N2 Separation with Minimum Thickness and High CO2 Permeance. ChemSusChem 2017, 10, 4014–4017. [Google Scholar] [CrossRef] [Green Version]
- Berned-Samatán, V.; Jiménez, S.; Rubio, C.; Téllez, C.; Coronas, J. Self-supported single-wall carbon nanotube buckypaper membranes applied to air and water fi ltration. J. Chem. Technol. Biotechnol. 2022, 98, 159–167. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Paseta, L.; Navarro, M.; Zornoza, B.; Téllez, C.; Coronas, J. Ultrapermeable Thin Film ZIF-8/Polyamide Membrane for H2/CO2 Separation at High Temperature without Using Sweep Gas. Adv. Mater. Interfaces 2018, 5, 1800647. [Google Scholar] [CrossRef]
- Tang, P.-H.H.; So, P.B.; Li, W.-H.H.; Hui, Z.-Y.Y.; Hu, C.-C.C.; Lin, C.-H.H. Carbon Dioxide Enrichment PEBAX/MOF Composite Membrane for CO2 Separation. Membranes 2021, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.C.; Lin, Y.Y.; Zhang, J.P.; Chen, X.M. Ligand-directed strategy for zeolite-type metal-organic frameworks: Zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.X.; Dai, Y.; Dong, C.Y.; Yang, X.C.; Xi, Y. Zeolite imidazolate framework (ZIF)-based mixed matrix membranes for CO2 separation: A review. J. Appl. Polym. Sci. 2020, 137, 48968. [Google Scholar] [CrossRef]
- Kattula, M.; Ponnuru, K.; Zhu, L.; Jia, W.; Lin, H.; Furlani, E.P. Designing ultrathin film composite membranes: The impact of a gutter layer. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Xie, K.; Nothling, M.D.; Gurr, P.A.; Tan, S.S.L.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrathin Metal–Organic Framework Nanosheets as a Gutter Layer for Flexible Composite Gas Separation Membranes. ACS Nano 2018, 12, 11591–11599. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Li, H.; Zhang, C.; Pan, H.; Zhang, Y.; Feng, X.; Tang, K.; Meng, J. A microporous polymer TFC membrane with 2-D MOF nanosheets gutter layer for efficient H2 separation. Sep. Purif. Technol. 2021, 261, 118283. [Google Scholar] [CrossRef]
- Ying, Y.; Yang, Z.; Shi, D.; Peh, S.B.; Wang, Y.; Yu, X.; Yang, H.; Chai, K.; Zhao, D. Ultrathin covalent organic framework film as membrane gutter layer for high-permeance CO2 capture. J. Memb. Sci. 2021, 632, 119384. [Google Scholar] [CrossRef]
- Gibson, P.; Schreuder-Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 469–481. [Google Scholar] [CrossRef]
- Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Recent trends in nanofibrous membranes and their suitability for air and water filtrations. Membranes 2011, 1, 232–248. [Google Scholar] [CrossRef]
- Rashid, M.H.-O.O.; Ralph, S.F. Carbon Nanotube Membranes: Synthesis, Properties and Future Filtration Applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutrisna, P.D.; Hou, J.; Li, H.; Zhang, Y.; Chen, V. Improved operational stability of Pebax-based gas separation membranes with ZIF-8: A comparative study of flat sheet and composite hollow fibre membranes. J. Memb. Sci. 2017, 524, 266–279. [Google Scholar] [CrossRef]
- Chen, F.; Dong, S.; Wang, Z.; Xu, J.; Xu, R.; Wang, J. Preparation of mixed matrix composite membrane for hydrogen purification by incorporating ZIF-8 nanoparticles modified with tannic acid. Int. J. Hydrogen Energy 2020, 45, 7444–7454. [Google Scholar] [CrossRef]
- Rojas, J.A.; Ardila-Rodríguez, L.A.; Diniz, M.F.; Gonçalves, M.; Ribeiro, B.; Rezende, M.C. Optimization of Triton X-100 removal and ultrasound probe parameters in the preparation of multiwalled carbon nanotube buckypaper. Mater. Des. 2019, 166, 107612. [Google Scholar] [CrossRef]
- Chew, S.Y.; Ng, S.H.; Wang, J.; Novák, P.; Krumeich, F.; Chou, S.L.; Chen, J.; Liu, H.K. Flexible free-standing carbon nanotube films for model lithium-ion batteries. Carbon 2009, 47, 2976–2983. [Google Scholar] [CrossRef]
- Berned-Samatán, V.; Rubio, C.; Galán-González, A.; Muñoz, E.; Benito, A.M.; Maser, W.K.; Coronas, J.; Téllez, C. Single-walled carbon nanotube buckypaper as support for highly permeable double layer polyamide/zeolitic imidazolate framework in nanofiltration processes. J. Memb. Sci. 2022, 652, 120490. [Google Scholar] [CrossRef]
- Valadez Sánchez, E.P.; Gliemann, H.; Haas-Santo, K.; Wöll, C.; Dittmeyer, R. ZIF-8 SURMOF Membranes Synthesized by Au-Assisted Liquid Phase Epitaxy for Application in Gas Separation. Chem. Ing. Tech. 2016, 88, 1798–1805. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Liu, X.; Benzaqui, M.; Gkaniatsou, E.; Orsi, A.; Lozinska, M.M.; Sicard, C.; Johnson, T.; Steunou, N.; Wright, P.A.; et al. Influence of Filler Pore Structure and Polymer on the Performance of MOF-Based Mixed-Matrix Membranes for CO2 Capture. Chem.—A Eur. J. 2018, 24, 7949–7956. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Izquierdo, L.; Malankowska, M.; Téllez, C.; Coronas, J. Phase inversion method for the preparation of Pebax® 3533 thin film membranes for CO2/N2 separation. J. Environ. Chem. Eng. 2021, 9, 105624. [Google Scholar] [CrossRef]
- Martínez-Izquierdo, L.; Téllez, C.; Coronas, J. Highly stable Pebax® Renew® thin-film nanocomposite membranes with metal organic framework ZIF-94 and ionic liquid [Bmim][BF4] for CO2 capture. J. Mater. Chem. A 2022, 10, 18822–18833. [Google Scholar] [CrossRef]
- James, J.B.; Lin, Y.S. Kinetics of ZIF-8 Thermal Decomposition in Inert, Oxidizing and Reducing Environments. J. Phys. Chem. C 2016, 120, 14015–14026. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Hou, L. Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM2.5 removal. RSC Adv. 2018, 8, 31471–31477. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Laínez, J.; Ballester-Catalán, M.; Javierre-Ortín, E.; Téllez, C.; Coronas, J. Pebax® 1041 supported membranes with carbon nanotubes prepared: Via phase inversion for CO2/N2 separation. Dalt. Trans. 2020, 49, 2905–2913. [Google Scholar] [CrossRef]
- Martínez-Izquierdo, L.; Perea-Cachero, A.; Malankowska, M.; Téllez, C.; Coronas, J. A comparative study between single gas and mixed gas permeation of polyether-block-amide type copolymer membranes. J. Environ. Chem. Eng. 2022, 10, 108324. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef]
- Elyasi Kojabad, M.; Momeni, M.; Babaluo, A.A.; Vaezi, M.J. PEBA/PSf Multilayer Composite Membranes for CO2 Separation: Influence of Dip Coating Parameters. Chem. Eng. Technol. 2020, 43, 1451–1460. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Mashhadikhan, S.; Mardassi, G.; Ebadi Amooghin, A.; Van der Bruggen, B.; Moghadassi, A. Aminosilane cross-linked poly ether-block-amide PEBAX 2533: Characterization and CO2 separation properties. Korean J. Chem. Eng. 2019, 36, 1339–1349. [Google Scholar] [CrossRef]
- Fu, Q.; Halim, A.; Kim, J.; Scofield, J.M.P.; Gurr, P.A.; Kentish, S.E.; Qiao, G.G. Highly permeable membrane materials for CO2 capture. J. Mater. Chem. A 2013, 1, 13769–13778. [Google Scholar] [CrossRef]
- Liu, M.; Xie, K.; Nothling, M.D.; Zu, L.; Zhao, S.; Harvie, D.J.E.; Fu, Q.; Webley, P.A.; Qiao, G.G. Ultrapermeable Composite Membranes Enhanced via Doping with Amorphous MOF Nanosheets. ACS Cent. Sci. 2021, 7, 671–680. [Google Scholar] [CrossRef]
- Ashtiani, S.; Sofer, Z.; Průša, F.; Friess, K. Molecular-level fabrication of highly selective composite ZIF-8-CNT-PDMS membranes for effective CO2/N2, CO2/H2 and olefin/paraffin separations. Sep. Purif. Technol. 2021, 274, 119003. [Google Scholar] [CrossRef]
- Feng, S.; Ren, J.; Zhao, D.; Li, H.; Hua, K.; Li, X.; Deng, M. Effect of poly (ethylene glycol) molecular weight on CO2/N2 separation performance of poly (amide-12-b-ethylene oxide)/poly (ethylene glycol) blend membranes. J. Energy Chem. 2019, 28, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Yang, X.; Zheng, Y.; He, X.; Chen, F.; Luo, J. Preparation of poly (ether-block-amide)/poly (amide-co-poly (propylene glycol)) random copolymer blend membranes for CO2/N2 separation. Polym. Eng. Sci. 2019, 59, E14–E23. [Google Scholar] [CrossRef]






| Time (days) | CO2 Permeance (GPU) | CO2/N2 Selectivity |
|---|---|---|
| 0 | 565 | 20.5 |
| 90 | 566 | 20.1 |
| 100 | 564 | 20.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berned-Samatán, V.; Téllez, C.; Coronas, J. Double-Layered Pebax® 3533/ZIF-8 Membranes with Single-Walled Carbon Nanotube Buckypapers as Support for Gas Separation. Membranes 2023, 13, 71. https://doi.org/10.3390/membranes13010071
Berned-Samatán V, Téllez C, Coronas J. Double-Layered Pebax® 3533/ZIF-8 Membranes with Single-Walled Carbon Nanotube Buckypapers as Support for Gas Separation. Membranes. 2023; 13(1):71. https://doi.org/10.3390/membranes13010071
Chicago/Turabian StyleBerned-Samatán, Víctor, Carlos Téllez, and Joaquín Coronas. 2023. "Double-Layered Pebax® 3533/ZIF-8 Membranes with Single-Walled Carbon Nanotube Buckypapers as Support for Gas Separation" Membranes 13, no. 1: 71. https://doi.org/10.3390/membranes13010071
APA StyleBerned-Samatán, V., Téllez, C., & Coronas, J. (2023). Double-Layered Pebax® 3533/ZIF-8 Membranes with Single-Walled Carbon Nanotube Buckypapers as Support for Gas Separation. Membranes, 13(1), 71. https://doi.org/10.3390/membranes13010071