1. Introduction
Membrane technology plays a crucial role in many separation processes, including wastewater treatment and drinking water production. The most commonly used membranes can be classified into two main families based on the material they are made of: ceramic and polymeric membranes. Each of them has its own advantages. Polymeric materials are more versatile in terms of shape and morphology to produce membranes with low-cost processes, which allows them to be tailored to each application, covering the whole range of pore sizes down to dense membranes [
1,
2]). Ceramic membranes, mainly composed of mineral oxides, have high thermal and chemical stability, making them suitable for a wide range of applications [
3]. Their high permeability and long operation lifespan before replacement characterize these membranes thanks to their easy cleaning and stability under harsh conditions. However, the energy-intensive, multi-step manufacturing process, which induces a high investment cost, remains an undeniable limitation to their industrial development, even though the life cycle analysis shows that they could be competitive in many applications [
4,
5].
Therefore, many efforts are devoted to reducing the overall manufacturing cost of ceramic membranes. A first approach is to replace expensive pure metal oxide powders, such as Al
2O
3, ZrO
2 or TiO
2, commonly used to prepare ceramic membranes [
6] with naturally occurring raw materials, such as clays [
7,
8,
9], phosphates [
10], kaolin [
11,
12] and fly ash [
13,
14]. Furthermore, it is generally accepted that sintering energy requiring very high temperatures contributes to about 60% of the overall manufacturing cost of ceramic membranes. A recent review describes strategies used to decrease sintering energy by lowering the temperature, process time and number of steps [
15].
Ceramic–polymer composite membranes are gaining interest because they can lead to a beneficial trade-off to obtain materials with improved properties by combining some advantages of both types of materials [
16]. For example, incorporating ceramic particles into a polymer matrix generally improves the membrane hydrophilicity, resulting in higher permeation flux and fouling resistance [
17], and increases its mechanical and thermal stability. It should be noted that the increase in membrane performance requires a high compatibility between the mineral particles and the polymer chains. Thus, a suspension of mineral particles in a polymer solution can lead to membranes with morphologies similar to those of polymer membranes using the non-solvent-induced phase inversion technique. In this promising one-step, low-cost approach, the polymer acts as a binder between the mineral particles, ensuring the mechanical stability of the resulting composite membrane. For instance, a suspension of alumina/polyethersulfone (ca 10/1 wt%) in N-methylpyrrolidone has been subjected to phase inversion using water as the polymer coagulation liquid [
18]. Morphologies ranging from a sponge-like structure to a finger-like structure have been obtained depending on the viscosity of the dope solution, adjusted by adding a varying amount of non-solvent. The same morphologies have been observed by other authors using similar preparation conditions in terms of suspension compositions and non-solvent mixtures [
19,
20].
Environmental constraints and the expected scarcity of fossil fuels make renewable polymers an alternative to conventional petroleum-based polymers. Many polysaccharides have film-forming properties that make them suitable for the preparation of membranes. In this context, we have undertaken a study on kaolin (KO) and chitosan (CS)-based composite membranes [
11,
21]. KO has been widely studied as a starting material for low-cost ceramic membranes due to its low sintering temperature and availability [
15,
22]. CS, extracted by alkaline deacetylation of chitin, the second most abundant polysaccharide on Earth, is considered a potential green polymer that can be used in many industrial fields. Indeed, the presence of reactive functions (hydroxyl and amino groups) makes it attractive for physicochemical interactions and subsequent chemical modifications. However, the low mechanical strength, stability and solubility of CS films at acidic pH generally limit their applications to dense membranes in aqueous-organic media [
23,
24]. Composite materials have been developed to overcome some of the limitations of CS, including in membranes [
25], adsorption materials to reduce water pollution [
26,
27] and biomedical applications [
28]. Cross-linking via reactive amino groups with various chemical agents is another strategy to stabilize CS films [
29,
30,
31,
32].
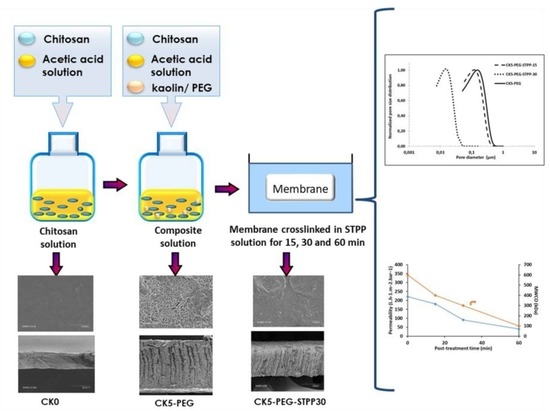
A previous study has reported the preparation of novel green KO/CS composite membranes by the phase inversion technique using only water as a solvent and non-solvent. In this case, the driving force of the phase inversion was achieved by the change in pH [
11]. The film cast in 0.1 M acetic acid was immersed in 1 M NaOH to induce CS phase separation. The obtained membranes showed the best mechanical properties for a KO:CS mass ratio of 55:45. The surface of the KO particles was negatively charged while the CS chains were positively charged due to protonation of the amine groups (pKa ca 6.5). The resulting ionic cross-linking reaction between CS and KO strongly reinforced the cohesion of the composite material. However, the low permeability observed and the low surface porosity of these composite membranes limit their applicability in filtration. An increase in permeability of about one order of magnitude could be obtained by incorporating in the suspension a small amount of water-soluble polyethylene glycol (PEG) as a pore-forming agent [
21]. This formulation resulted in membranes with higher wettability and a very porous finger-like morphology, accounting for the increased permeability.
The objective of this report is to investigate the cross-linking ability of CS in composite membranes to improve their chemical stability, mainly in acidic media. The protonation of amine groups leads to the partial dissolution of CS in water. It has been shown in our previous work that ionic interactions with KO shift the dissolution to a lower pH value, but not enough to allow the use of such membranes in aqueous applications [
11,
21]. CS can be cross-linked covalently or ionically. Harmful chemicals such as glutaraldehyde and glyoxal are most often used for covalent cross-linking [
33]. An alternative is to use genipin, a naturally occurring chemical cross-linker [
31]. On the other hand, physical cross-linking agents such as low-molecular-weight polyanions, including citrate, tripolyphosphate (STPP) and sulfate, have also been used to circumvent this problem [
27,
32,
34,
35]. STPP was chosen for this study because it is known to possess a high charge density and to be able to diffuse into the CS network during the cross-linking reaction. Thus, the fabrication of a CS/KO/PEG composite cross-linked with STPP seems promising to address applications in membrane technology. Another point to mention is that the cross-linking density leads to a tightening of the pore structure [
36,
37]. Therefore, this work aims to investigate whether it is possible to control the pore size by altering the cross-linking time and thus obtain a range of CS/KO composite membranes of variable selectivity.
The effect of cross-linking density on the properties of the resulting composite membranes was investigated in terms of surface chemistry, structural morphology and pore size distribution (PSD) using techniques such as water contact angle, zeta potential, ATR-FTIR and microscopic observation. In addition, the thermal, mechanical and chemical stability levels of the prepared membranes were also determined. Finally, filtration properties in terms of pure water permeability and molecular weight cut-off (determined from PEG standards rejection) were presented.
3. Results and Discussion
3.1. Effect of Cross-Linking Reaction Time on Membrane Morphology
Membrane morphology is a very important feature as it influences many membrane properties.
Figure 1 shows the evolution of the surface morphology of the composite membranes in the presence of the pore-forming agent PEG and after a 30-minure post-treatment with STPP. A uniform, smooth and flat surface with no detectable pores was observed in the case of the pristine CS membrane (CK0). In contrast, the composite membranes showed rougher surfaces with apparent KO particles. However, the penetration and intercalation of CS chains into the KO layers gives rise to a cohesive three-dimensional structure. A well-defined pore structure appears when the starting suspension formulation contains PEG, indicating that it plays the role of a pore-forming agent through its interaction with CS. Pore formation is then related to the release of PEG to the external solution during phase separation [
21]. As can be seen in
Figure 1, post-treatment with STPP led to a tightening of the surface pores.
The effect of cross-linking on the top surface porosity and bulk morphology is further examined in
Figure 2 (left), which compares the surface morphology of the untreated membrane (CK5-PEG) with that of the membranes that underwent a post-treatment with STPP for 15, 30 and 60 min. As can be seen, cross-linking significantly affected the top surface pore size and pore density, indicating that negatively charged STPP can easily diffuse into the interpenetrated positively charged CS chains. After immersing the CK5-PEG membrane in STPP solution, a decrease in pore size could be observed with reaction time, such that only a few pores could be detected in SEM micrographs after 60 min of reaction. It can also be noted that network reorganization requires some induction time. Indeed, after 15 min of contact, little change in surface pore size and density was observed with the starting membrane. In contrast, a noticeable reduction in surface pore size occurred after 30 min of immersion in the cross-linking bath (CK5-PEG-STPP30). These operating conditions allowed the preparation of composite membranes with circular, well-defined and much smaller surface pores. However, longer reaction times led to pore clogging and the appearance of some holes around the KO particles (CK5-PEG-STPP60). It can be concluded that the cross-linking makes the membrane surface evolve from a foam morphology to a dense layer. This result is in agreement with previous reports that have shown that long reaction times result in increased pore densification of a CS membrane due to the higher cross-linking density [
37].
Figure 2 (right) shows micrographs of the corresponding membrane cross-sections. SEM observation revealed that the introduction of PEG into the KO/CS suspension resulted in a highly porous sponge-like structure with interconnected pores on the surface and the appearance of finger-like structures underneath. As mentioned above, similar structures characteristic of the non-solvent phase inversion method have been found for other types of composite membranes [
6], and the mechanism of their formation has been explained by different authors [
18,
19]. Cross-linking resulted in membrane thickening without affecting the finger-like structure significantly, except in the case of the longer reaction time (CK5-PEG-STPP60). The gradual penetration of STPP and intercalation between CS chains through electrical interactions are assumed to lead to the expansion of the polymer network, resulting in thickening of the top layer and that of the membrane cross-section.
Figure 3 shows the evolution of the average thickness of the composite membrane and that of the surface layer above the finger-like structure determined from the SEM images, as a function of contact time with the STPP solution.
A two-step process could explain these findings: first, a rapid diffusion of STPP inside the membrane resulting in a steep increase in its total thickness, followed by reorganization of the CS polymer chains, probably due to an exchange of the electrical interactions between ammonium groups with KO by those with STPP. This second step corresponds to the intensification of the cross-linking reaction and is associated with densification of the polymeric network and a steady increase in the thickness of the top layer.
Figure 4 shows the PSD determined by image analysis of SEM photos of the three membrane top surfaces prepared with respective cross-linking reaction times of 0, 15 and 30 min (
Figure 2, left). Note that the image analysis of the membrane surfaces cross-linked for 60 min did not yield significant values due to the low number of measurable pores.
3.2. Pore Size Distribution (PSD)
As discussed in the previous section, the surface size decreased with increasing the cross-linking time. However, it can be observed in
Figure 4 that the PSD remained similar for all samples. It only shifted as the cross-linking reaction increased. The peak maximum was taken as the characteristic pore size value for the prepared composite membranes. It varied from about 160 nm for the non-cross-linked CK5-PEG membrane to about 15 nm for a membrane cross-linked for 30 min (CK5-PEG-STPP30). As mentioned before, the pore size changed little during the first 15 min of the reaction (from 160 nm to about 125 nm for CK5-PEG-STPP15), in agreement with the assumption of an induction time mainly related to the movement of the polymer chains to allow the cross-linking reaction to occur.
In conclusion, cross-linking by electric interactions between the anionic phosphate groups of STPP and the cationic protonated amino groups of CS led to dramatic morphological changes in the composite membranes—in particular, a densification of the top surface layer resulting in a significant reduction in pore size. These two parameters can have a significant influence on the surface physicochemical properties and the operating performances of the membranes (see the following sections). Thus, a variety of membranes with pore sizes ranging from microfiltration to ultrafiltration could be prepared by varying the cross-linking conditions by STTP. The discussion below focuses primarily on the properties of CK5-PEG-STTP30, which represents the composite membrane most impacted by cross-linking.
3.3. Surface Chemistry of the Composite Membranes
Figure 5 presents the elemental composition of the surfaces of the pristine CS membranes, the CK5 composite and the cross-linked CK5-PEG-STPP30 composite, determined by EDX microanalysis. The CS membrane composition showed mainly the presence of C, N and O elements, while additional signals corresponding to Si and Al elements were detected in the CK5 composite membrane. A new peak corresponding to phosphorus could be observed in the cross-linked membrane, confirming the incorporation of STPP in the composite membrane.
The ATR-FTIR spectra of STPP powder and the non-cross-linked and cross-linked composite membranes were compared to highlight their signature peaks and to investigate the chemical interactions taking place between the composite membrane components (
Figure 6). One of the characteristic peaks of STPP powder found at 1212 cm
−1 and attributed to the antisymmetric stretching vibrations of the PO
2- group could be observed in the spectrum of CK5-PEG-STPP30, while it was not present in that of CK5-PEG, indicating that the cross-linking agent was indeed introduced into the membranes. In addition, another band appeared in the spectrum of the cross-linked membrane at 1540 cm
−1, attributed to the antisymmetric deformation N-H vibration of the NH
3+ ion related to the protonated CS. Under the acidic conditions of cross-linking, strong electrical interactions between NH
3+ cations and PO
2- anions can occur, causing a reduction in the intensity of the STPP peak at 1212 cm
−1 compared to that of pristine STPP powder [
35].
3.4. Thermal and Chemical Stability of the Composite Membranes
The thermal stability of the prepared composite membranes was determined by TGA.
Figure 7 presents the typical thermograms obtained for the STPP powder and the non-cross-linked (CK5-PEG) and 30-minute cross-linked (CK5-PEG-STPP30) composite membranes. As can be seen, STPP can be considered thermally stable up to 800 °C. Three weight losses can be observed in the TGA curves of both CK5-PEG and CK5-PEG-STPP30. The first one between 50 and 150 °C is attributed to the release of water molecules more or less adsorbed within the composite membrane. The second weight loss between 200 and 400 °C corresponds to the partial decomposition of the CS and remaining PEG chains, while the third between 400 and 600 °C is assumed to result from the simultaneous dehydroxylation of kaolinite and final degradation of the CS chains [
21]. Comparing the thermograms of these two samples, a lowering of about 50 °C can be observed for the decomposition temperature of the polymer moiety from 298 to 250 °C as a consequence of the cross-linking with STPP. A difference of the same order of magnitude can also be seen in the case of the inorganic part from 569 to 494 °C. The competition between the electrical interactions of CS with KO and those between CS and STPP required for the polymer cross-linking probably explains the observed decrease in thermal stability. Finally, the difference in residual weight at 800 °C of about 10% of the total weight between CK5-PEG and CK5-PEG-STPP30 reveals the amount of STPP included in the cross-linked membrane.
Resistance to water washout is an important parameter to consider when using these composite membranes in water treatment applications. Protonation of the amine groups in an acidic environment results in partial dissolution of CS, causing deterioration of the membrane material. Therefore, the water solubilization (WS) of the pristine CS membrane (CK0) and the non-cross-linked and cross-linked membranes under different pH conditions (pH = 2, 4 and 9 and at the natural pH of deionized water (6.2)) was investigated to examine their chemical stability in aqueous media (
Table 1). As expected, the pure CS membrane had low chemical stability and dissolved at pH values below neutrality, while the composite membranes retained their original state at this pH. The association with KO prevented the dissolution of CS, confirming that the membrane did not correspond to a simple mixture but resulted from a strong interaction between the different components.
However, these interactions were weakened by decreasing the pH, which eventually led to the complete dissolution of the membranes at pH 4. Therefore, stabilization of the composite membranes at acidic pH is crucial for applications in aqueous media. The obtained data in
Table 1 show that cross-linking with STPP can solve this issue. Indeed, the cross-linked composite membrane CK5-PEG-STPP30 was stable at acidic pH even when immersed in a medium with pH 2. The compact network formed by high numbers of inter-chain bonds between CS and STPP led to the formation of a three-dimensional network structure and the loss of chain mobility responsible for the improved chemical stability of the resulting membranes. Moreover, cross-linking greatly reduces the number of protonated amine groups available to induce CS dissolution.
3.5. Mechanical Properties of the Composite Membranes
Good mechanical properties are one of the requirements for practical applications of the membranes. Therefore, the tensile strength and elongation at break were measured in the wet state (
Table 2). These properties are closely correlated with the membrane structure, as the results showed that the mechanical properties of the STPP-cross-linked composite membranes were affected by the cross-linking time. The tensile strength values increased significantly by lengthening the cross-linking time, with the CK5-PEG-STPP60 sample showing a value more than twice that of the non-cross-linked membrane. In a previous section, it was shown that the morphology of the composite membrane was profoundly altered upon cross-linking with STPP. The formation of the three-dimensional network between CS and STPP results in an increase in membrane thickness and densification of the structure. The increase in the mechanical strength of the cross-linked membranes can then be explained by the restriction of CS chain movements and the decrease in pore volume.
However, the opposite trend was observed for elongation at break. The elongation at break of the membranes decreased with increasing the cross-linking time, indicating an increase in the stiffness and brittleness of the membranes. This can again be explained by the increase in the number of inter-chain bonds as the reaction proceeded, reducing the flexibility of the network and, thus, the plasticity of the material. From these values, it is obvious that cross-linking plays a crucial role in improving the mechanical strength and reducing the elongation at break of the composite membranes. Despite these variations, the mechanical properties obtained remain well suited to the operating conditions of microfiltration and ultrafiltration.
3.6. Surface Properties of the Composite Membranes
The interfacial properties between the membrane and the feed solution strongly influence the performance of filtration in aqueous media. The hydrophilicity and surface charge can be determined by WCA and zeta potential measurements, respectively.
Table 2 shows the results of WCA measurements for the different prepared composite membranes, illustrating the effect of cross-linking on surface wettability. The obtained WCA values show a steady increase with treatment time, indicating that the surface became less and less hydrophilic. However, the difference with respect to the starting membrane remained small (ca 10°). This result is in agreement with the literature. The introduction of a CS cross-linking step has been reported to decrease the hydrophilic nature of the membrane [
32]. This effect can be explained by the increase in the rigidity of the CS backbone and the reduction in the pore size, which prevent the access of water molecules to the CS polar group and also the decrease in the number of hydrophilic functions (amine and hydroxyl) available to yield solvation interactions with water.
The surface charge of CK5-PEG-STPP30 was determined in terms of zeta potential as a function of pH (
Figure 8). The isoelectric point was found to be close to pH 7, meaning that the membrane surface was positively charged for pH values below 7 and negatively charged above. The protonation of the amine functions of CS (pKa about 6) is responsible for the positive value of the zeta potential in acidic media, whereas the negative charges of KO and STPP become predominant in basic media due to the deprotonation of the amine functions. It is noticeable that the zeta potential value (maximum about 15 mV) remained low over the whole pH range, confirming that the ionic functions were mainly involved in the interactions between the components of the composite membrane.
3.7. Permeation Properties of the Composite Membranes
Permeation properties, namely productivity and selectivity, were evaluated in terms of PWP and MWCO. It has been shown in previous work (Rekik et al., 2019) that the addition of PEG to a KO/CS suspension strongly improves the water permeability of the composite membranes obtained with this formulation owing to the sponge-like morphology of the surface layer and the presence of a finger-like through structure, as observed in
Figure 2.
Figure 9 shows the effect of cross-linking time on
PWP and
MWCO values. As can be seen, the
PWP values decreased with the reaction advancement. The aforementioned induction period of approximately 15 min led to only a slight decrease in water permeability despite the increased membrane thickness, confirming the previous findings. On the other hand, the reduction in permeability was more pronounced for longer reaction times. These results obtained under realistic membrane operating conditions are in agreement with the local determinations of morphological changes (pore size and top layer thickness) from SEM micrographs in
Section 3.1 and
Section 3.2. Clearly, the cross-linked composite membranes exhibited a more pronounced barrier to the passage of water molecules, resulting in a decrease in permeation flux. Interestingly, an excellent correlation was found between the PWP and the MWCO data determined for the corresponding membranes (
Figure 9). It can be concluded that the dominant parameter accounting for the decrease in permeability is the progressive reduction in pore size by cross-linking with STPP. Other factors such as increased surface layer thickness leading to enhanced tortuosity may also contribute to the decrease in PWP [
36]. Furthermore, the decrease in PWP as a function of cross-linking extent is also in good agreement with the increase in WCA values from CK5-PEG-STPP15 to CK5-PEG-STPP60.
The selectivity of the composite membranes in terms of MWCO confirms that the potential field of application can cover the range from microfiltration to ultrafiltration. Pore size tuning by STPP cross-linking could then allow the preparation of KO/CS composite membranes with specified properties in terms of productivity and selectivity by adjusting the reaction conditions.







 ) for CK5-PEG-STPP30 composite membrane.
) for CK5-PEG-STPP30 composite membrane.