Recursive Alterations of the Relationship between Simple Membrane Geometry and Insertion of Amphiphilic Motifs
Abstract
:1. Introduction
2. Different Modes of Interaction
2.1. Two Insertion Regimes: MCS and MCG
2.2. Geometric Models for Insertion of AIMs
2.3. Geometric LPD-Model 1: Saturation of Static Membranes
2.4. Geometric LPD-Model 2: Saturation of Dynamic Membranes
3. Thermodynamic Insertion-Model
3.1. Basic Intrinsic Properties of Curved Membranes
3.2. Saturation of Insertion in Fixed Membrane Morphologies
4. Recursive Changes of Membrane Morphology
4.1. Fission in a Closed Uncoupled Compartment
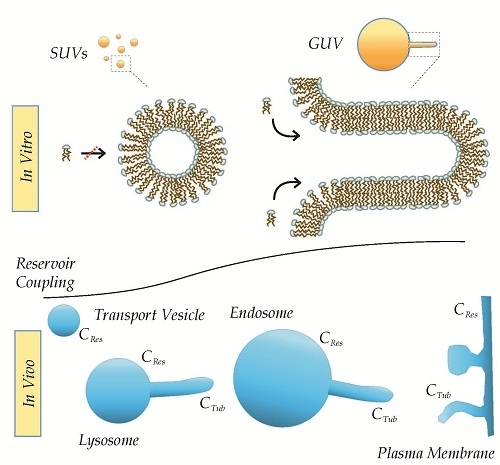
4.2. Recursive Morphology Change in Open Coupled Systems
5. Summary
6. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Ambroggio, E.; Sorre, B.; Bassereau, P.; Goud, B.; Manneville, J.B.; Antonny, B. ArfGAP1 generates an Arf1 gradient on continuous lipid membranes displaying flat and curved regions. EMBO J. 2010, 29, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Drin, G.; Levi, S.; Rawet, M.; Cassel, D.; Bigay, J.; Antonny, B. Two lipid-packing sensor motifs contribute to the sensitivity of arfgap1 to membrane curvature. Biochemistry 2007, 46, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Schmick, M.; Bastiaens, P.I. The interdependence of membrane shape and cellular signal processing. Cell 2014, 156, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Antonny, B.; Burd, C.; De Camilli, P.; Chen, E.; Daumke, O.; Faelber, K.; Ford, M.; Frolov, V.A.; Frost, A.; Hinshaw, J.E.; et al. Membrane fission by dynamin: What we know and what we need to know. EMBO J. 2016, 35, 2270–2284. [Google Scholar] [CrossRef] [PubMed]
- Qualmann, B.; Koch, D.; Kessels, M.M. Let’s go bananas: Revisiting the endocytic bar code. EMBO J. 2011, 30, 3501–3515. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A. Phosphoinositide recognition domains. Traffic (Copenhagen, Denmark) 2003, 4, 201–213. [Google Scholar] [CrossRef]
- Frost, A.; Perera, R.; Roux, A.; Spasov, K.; Destaing, O.; Egelman, E.H.; De Camilli, P.; Unger, V.M. Structural basis of membrane invagination by F-BAR domains. Cell 2008, 132, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Mim, C.; Cui, H.; Gawronski-Salerno, J.A.; Frost, A.; Lyman, E.; Voth, G.A.; Unger, V.M. Structural basis of membrane bending by the n-bar protein endophilin. Cell 2012, 149, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Niwa, H.; Tsujita, K.; Suetsugu, S.; Nitta, K.; Hanawa-Suetsugu, K.; Akasaka, R.; Nishino, Y.; Toyama, M.; Chen, L.; et al. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 2007, 129, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Bigay, J.; Antonny, B. Curvature, lipid packing, and electrostatics of membrane organelles: Defining cellular territories in determining specificity. Dev. Cell 2012, 23, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Bhatia, V.K.; Gether, U.; Stamou, D. Bar domains, amphipathic helices and membrane-anchored proteins use the same mechanism to sense membrane curvature. FEBS Lett. 2010, 584, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Vamparys, L.; Gautier, R.; Vanni, S.; Bennett, W.F.; Tieleman, D.P.; Antonny, B.; Etchebest, C.; Fuchs, P.F. Conical lipids in flat bilayers induce packing defects similar to that induced by positive curvature. Biophys. J. 2013, 104, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.; Vamparys, L.; Gautier, R.; Drin, G.; Etchebest, C.; Fuchs, P.F.; Antonny, B. Amphipathic lipid packing sensor motifs: Probing bilayer defects with hydrophobic residues. Biophys. J. 2013, 104, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Lyman, E.; Voth, G.A. Mechanism of membrane curvature sensing by amphipathic helix containing proteins. Biophys. J. 2011, 100, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.; Hirose, H.; Barelli, H.; Antonny, B.; Gautier, R. A sub-nanometre view of how membrane curvature and composition modulate lipid packing and protein recruitment. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Hatzakis, N.S.; Bhatia, V.K.; Larsen, J.; Madsen, K.L.; Bolinger, P.Y.; Kunding, A.H.; Castillo, J.; Gether, U.; Hedegard, P.; Stamou, D. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol. 2009, 5, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Magdeleine, M.; Gautier, R.; Gounon, P.; Barelli, H.; Vanni, S. A filter at the entrance of the Golgi that selects vesicles according to size and bulk lipid composition. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Campelo, F.; McMahon, H.T.; Kozlov, M.M. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys. J. 2008, 95, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Farsad, K.; Ringstad, N.; Takei, K.; Floyd, S.R.; Rose, K.; De Camilli, P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 2001, 155, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Slepnev, V.I.; Haucke, V.; De Camilli, P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1999, 1, 33–39. [Google Scholar] [PubMed]
- Kabelka, I.; Vacha, R. Optimal conditions for opening of membrane pore by amphiphilic peptides. J. Chem. Phys. 2015, 143. [Google Scholar] [CrossRef] [PubMed]
- Boucrot, E.; Pick, A.; Camdere, G.; Liska, N.; Evergren, E.; McMahon, H.T.; Kozlov, M.M. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent bar domains. Cell 2012, 149, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Capraro, B.R.; Zhu, C.; Das, S.L. Thermodynamics and mechanics of membrane curvature generation and sensing by proteins and lipids. Annu. Rev. Phys. Chem. 2011, 62, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.; Evans, P.R.; McMahon, H.T. Bar domains as sensors of membrane curvature: The amphiphysin bar structure. Science (New York, N.Y.) 2004, 303, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, S.; Toyooka, K.; Senju, Y. Subcellular membrane curvature mediated by the bar domain superfamily proteins. Semin. Cell Dev. Biol. 2010, 21, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Heimburg, T. Thermal Biophysics of Membranes; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- McMahon, H.T.; Boucrot, E. Membrane curvature at a glance. J. Cell Sci. 2015, 128, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Hed, G.; Safran, S.A. Initiation and dynamics of hemifusion in lipid bilayers. Biophys. J. 2003, 85, 381–389. [Google Scholar] [CrossRef]
- Friedman, J.R.; Voeltz, G.K. The ER in 3D: A multifunctional dynamic membrane network. Trends Cell Biol. 2011, 21, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Gallon, M.; Cullen, P.J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 2015, 43, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Luzio, J.P.; Poupon, V.; Lindsay, M.R.; Mullock, B.M.; Piper, R.C.; Pryor, P.R. Membrane dynamics and the biogenesis of lysosomes. Mol. Membr. Biol. 2003, 20, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Raucher, D.; Sheetz, M.P. Characteristics of a membrane reservoir buffering membrane tension. Biophys. J. 1999, 77, 1992–2002. [Google Scholar] [CrossRef]
- Roux, A.; Cappello, G.; Cartaud, J.; Prost, J.; Goud, B.; Bassereau, P. A minimal system allowing tubulation with molecular motors pulling on giant liposomes. Proc. Natl. Acad. Sci. USA 2002, 99, 5394–5399. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.K.; Madsen, K.L.; Bolinger, P.Y.; Kunding, A.; Hedegard, P.; Gether, U.; Stamou, D. Amphipathic motifs in bar domains are essential for membrane curvature sensing. EMBO J. 2009, 28, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.B.; Jensen, M.B.; Bhatia, V.K.; Pedersen, S.L.; Bjornholm, T.; Iversen, L.; Uline, M.; Szleifer, I.; Jensen, K.J.; Hatzakis, N.S.; et al. Membrane curvature enables n-ras lipid anchor sorting to liquid-ordered membrane phases. Nat. Chem. Biol. 2015, 11, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, A.; Christensen, S.M.; Tkach, V.; Stamou, D. Geometrical membrane curvature as an allosteric regulator of membrane protein structure and function. Biophys. J. 2014, 106, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.P.; Kasemo, B. Adsorption of proteins on a lipid bilayer. Eur. Biophys. J. 2010, 39, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.R.; Goncalves da Silva, A.M.; Fedorov, A.; Futerman, A.H.; Prieto, M.; Silva, L.C. Influence of intracellular membrane ph on sphingolipid organization and membrane biophysical properties. Langmuir 2014, 30, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Honerkamp-Smith, A.R.; Veatch, S.L.; Keller, S.L. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta 2009, 1788, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, W. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Naturforsch. Teil C 1973, 28, 693–703. [Google Scholar] [CrossRef]
- Polozov, I.V.; Anantharamaiah, G.M.; Segrest, J.P.; Epand, R.M. Osmotically induced membrane tension modulates membrane permeabilization by class l amphipathic helical peptides: Nucleation model of defect formation. Biophys. J. 2001, 81, 949–959. [Google Scholar] [CrossRef]
- Kozlov, M.M.; Campelo, F.; Liska, N.; Chernomordik, L.V.; Marrink, S.J.; McMahon, H.T. Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 2014, 29, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Evergren, E.; Golushko, I.; Prevost, C.; Renard, H.F.; Johannes, L.; McMahon, H.T.; Lorman, V.; Voth, G.A.; Bassereau, P. How curvature-generating proteins build scaffolds on membrane nanotubes. Proc. Natl. Acad. Sci. USA 2016, 113, 11226–11231. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Voth, G.A.; Callan-Jones, A.; Bassereau, P. When physics takes over: Bar proteins and membrane curvature. Trends Cell Biol. 2015, 25, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Shahalami, M.; Wang, L.; Wu, C.; Masliyah, J.H.; Xu, Z.; Chan, D.Y. Measurement and modeling on hydrodynamic forces and deformation of an air bubble approaching a solid sphere in liquids. Adv. Colloid Interface Sci. 2015, 217, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Drin, G.; Antonny, B. Amphipathic helices and membrane curvature. FEBS Lett. 2010, 584, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.G.; Mills, I.G.; Peter, B.J.; Vallis, Y.; Praefcke, G.J.; Evans, P.R.; McMahon, H.T. Curvature of clathrin-coated pits driven by epsin. Nature 2002, 419, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Futerman, A.H.; van Meer, G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 2004, 5, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Chernomordik, L.V.; Kozlov, M.M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003, 72, 175–207. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Martens, G.J.; Huttner, W.B. Secretory granule biogenesis: Rafting to the snare. Trends Cell Biol. 2001, 11, 116–122. [Google Scholar] [CrossRef]
- Safran, S.A.; Kuhl, T.L.; Israelachvili, J.N. Polymer-induced membrane contraction, phase separation, and fusion via marangoni flow. Biophys. J. 2001, 81, 659–666. [Google Scholar] [CrossRef]
- Kukulski, W.; Schorb, M.; Kaksonen, M.; Briggs, J.A. Plasma membrane reshaping during endocytosis is revealed by time-resolved electron tomography. Cell 2012, 150, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, S.; Roche, M.; Cantat, I.; Saint-Jalmes, A. Soluble surfactant spreading: How the amphiphilicity sets the marangoni hydrodynamics. Phys. Rev. E 2016, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, C.; Kuo, C.J.; Robustelli, J.; Baumgart, T. The n-terminal amphipathic helix of endophilin does not contribute to its molecular curvature generation capacity. J. Am. Chem. Soc. 2016, 138, 14616–14622. [Google Scholar] [CrossRef] [PubMed]












| Relative Change | A (1 × A) | B (2 × A) | C (3 × A) |
|---|---|---|---|
| 1 | |||
| NaN | 0.87 | 0.95 | |
| 1 | 1.22 | 1.43 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madsen, K.L.; Herlo, R. Recursive Alterations of the Relationship between Simple Membrane Geometry and Insertion of Amphiphilic Motifs. Membranes 2017, 7, 6. https://doi.org/10.3390/membranes7010006
Madsen KL, Herlo R. Recursive Alterations of the Relationship between Simple Membrane Geometry and Insertion of Amphiphilic Motifs. Membranes. 2017; 7(1):6. https://doi.org/10.3390/membranes7010006
Chicago/Turabian StyleMadsen, Kenneth Lindegaard, and Rasmus Herlo. 2017. "Recursive Alterations of the Relationship between Simple Membrane Geometry and Insertion of Amphiphilic Motifs" Membranes 7, no. 1: 6. https://doi.org/10.3390/membranes7010006
APA StyleMadsen, K. L., & Herlo, R. (2017). Recursive Alterations of the Relationship between Simple Membrane Geometry and Insertion of Amphiphilic Motifs. Membranes, 7(1), 6. https://doi.org/10.3390/membranes7010006