Poly(vinylbenzylchloride) Based Anion-Exchange Blend Membranes (AEBMs): Influence of PEG Additive on Conductivity and Stability
Abstract
:1. Introduction
- The ionic conductivity of AEMs is significantly lower than that of CEMs despite having comparable ion exchange capacity (IEC), which is partly due to the fact that most AEM types have a hydrocarbon backbone, which is significantly less hydrophobic than, for example, the perfluorinated polymer main chain of the perfluorinated membranes of the Nafion® (Fayetteville, NC, USA) type, leading to a smaller separation between ionic groups and the polymer backbone and, therefore, to lower ionic conductivities due to the lower local density of the anion-exchange groups, particularly since in most AEM types the cationic head groups are connected to the polymer backbone only via a CH2 (benzylic) bridge [10] which hinders clustering of the anion-exchange groups.
- search for alternative fixed cations;
- chemical and/or physical cross-linking; and
- embedding of the anion-exchange polymers in an inert polymer matrix.
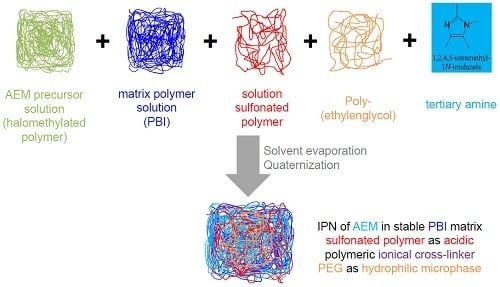
- (a)
- Three-component blends (after quaternization of the chlormethyl groups with the tertiary N-basic compounds) consisting of PVBCl, a sterically hindered tertiary N base, the polybenzimidazoles PBIOO or F6PBI, and a nonfluorinated and a partially fluorinated aromatic sulfonated polyether as ionic cross-linker (Figure 1a);
- (b)
- Four-component blends (after quaternization of the chlormethyl groups with the tertiary N-basic compounds) consisting of PVBCl, a sterically hindered tertiary N base, PBIOO, a nonfluorinated aromatic sulfonated polyethersulfone polymer as ionic cross-linker, and poly(ethylenglycol)s (PEGs) with different chain lengths and epoxide groups at the end of the PEG chain for anchoring in the PBI matrix by reacting the epoxy groups with the N–H groups of the imidazole moiety (Figure 1b). The reaction of the epoxy end groups of the PEG with the N-H groups of the PBI blend component is illustrated in Figure 2.
2. Results and Discussion
2.1. Membrane Properties
2.1.1. Brief Discussion of the Physico-Chemical Properties of the AEBMs
2.1.2. Membrane Conductivities of the AEBMs as a function of Temperature
- Membranes with the same calculated IECs (IECcalc = 2.3 meq/g), but with or without PEGs (NF-AEBM 2179B without PEG, and 2176 with PEG, see Figure 7).
- Membranes with different calculated IECs, but the same PBI:PEG molar ratios (NF-AEBMs 2176 and 2190A, see Figure 8).
- Membranes with the same composition, but different PEG molecular weights (NF-AEBM 2175 with PEG500, and NF-AEBM 2176 with PEG6000, see Figure 9)
- Conductivities of NF-AEBMs in comparison with F-AEBMs (membranes 2264/2246, 2261/2258 and 2262/2259 with the calculated IECscalc 2.0, 2.3 and 2.6, respectively, where the IEC variation was obtained by varying the TMIm-quaternized PVBCl content in the blend (Figure 11).
2.1.3. Thermal Stability of the AEBMs
- water evaporating from the membrane(4000 to 3400 cm−1)
- onset of CO2 development indicating the beginning degradation of the blend membrane, probably originating from the PEG blend component (the TGA-FTIR coupling experiment of pure PEG-diepoxide also showed an onset of CO2 formation in the same temperature range).
3. Materials and Methods
3.1. Materials
3.2. Membrane Preparation and Posttreatment
- The membranes were immersed in 10% ethanolic solutions of different tertiary amines. Most of the membranes were posttreated with 1,2,4,5-tetramethylimidazole (TMIm) to complete the quaternization reaction.
- The membranes were immersed in 10% NaCl solution for 48 h at 90 °C.
- The membranes were washed thoroughly with DI water and stored in DI water at 60 °C for 48 h.
- The membranes were soaked in an aqueous 1 M KOH solution at 90 °C for 10 days. Some of the membranes were stored in a 1 M KOH solution at 90 °C for 20 and 30 days, respectively.
- To determine the effectiveness of the reaction of the PEG’s epoxy end groups with the N–H groups of the PBI, a 10 wt % DMSO solution of pure PBI (80 wt %) was mixed with the PEG (PEG500, 20 wt %), before casting a membrane from this mixture. After membrane formation, the blend membrane was extracted with DMAc to determine the extent of cross-linking within the blend. According to the results the DMAc residual after extraction was nearly 100%, proving the completeness of cross-linking of the PBI with the epoxide end groups which confirms the covalent cross-linking of PBI with bisphenol A-bisepoxide and other bisepoxide compounds [51].
3.3. Membrane Characterization
3.3.1. Ion-Exchange Capacity (IEC)
3.3.2. Ionic Conductivity
3.3.3. Gel Content
3.3.4. Water Uptake
3.3.5. Thermal Stability
3.3.6. Alkaline Stability
4. Conclusions
Author Contribution
Conflicts of Interest
References
- Piana, M.; Boccia, M.; Filpi, A.; Flammia, E.; Miller, H.A.; Orsini, M.; Salusti, F.; Santiccioli, S.; Ciardelli, F.; Pucci, A. H2/air alkaline membrane fuel cell performance and durability, using novel ionomer and non-platinum group metal cathode catalyst. J. Power Sources 2010, 195, 5875–5881. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced alkaline water electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Yang, Z. Membrane Development for Vanadium Redox Flow Batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Liew, K.B.; Ismail, M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review Renew. Sustain. Energy Rev. 2013, 28, 575–587. [Google Scholar] [CrossRef]
- Gellett, W.; Schumacher, J.; Kesmez, M.; Le, D.; Minteer, S.D. High Current Density Air-Breathing Laccase Biocathode. J. Electrochem. Soc. 2010, 157, B557–B562. [Google Scholar] [CrossRef]
- Sandeaux, J.; Sandeaux, R.; Gavach, C. Competition between the electrotransports of acetate and chloride ions through a polymeric anion-exchange membrane. J. Memb. Sci. 1991, 59, 265–276. [Google Scholar] [CrossRef]
- Kliber, S.; Wisniewski, J.A. Removal of bromate and associated anions from water by Donnan dialysis with anion-exchange membrane. Desal. Water Treatm. 2011, 35, 158–163. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Erigene, B.; Mondal, A.N.; Hossain, M.M.; Khan, M.I.; Afsar, N.U.; Liang, G.; Wu, L.; Xu, T. Imidazolium functionalized anion exchange membrane blended with PVA for acid recovery via diffusion dialysis process. J. Memb. Sci. 2016, 497, 209–215. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; Xu, T.; Zhuang, L. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Slade, R.C.T.; Varcoe, J.R. Prospects for alkaline anion-exchange membranes in low temperature fuel cells. Fuel Cells 2005, 5, 187–200. [Google Scholar]
- Nunez, S.A.; Hickner, M.A. Quantitative H-1NMR Analysis of Chemical Stabilities in Anion-Exchange Membranes. ACS Macro Lett. 2013, 2, 49–52. [Google Scholar] [CrossRef]
- Fuson, R.C.; McKeever, C.H. Chloromethylation of Aromatic Compounds. In Organic Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1942; pp. 63–90. [Google Scholar]
- Zschocke, P.; Quellmalz, D. Novel ion-exchange membranes based on an aromatic polyethersulfone. J. Memb. Sci. 1985, 22, 325–332. [Google Scholar] [CrossRef]
- Wohl, A. Brominised unsaturated compounds with N-bromo-acetamide, a report on the knowledge of the progression of chemical processes. Ber. Dt. Chem. Ges. 1919, 52, 51–63. [Google Scholar] [CrossRef]
- Liska, J.; Borsig, E.; Tkac, I. A route to preparation of bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide). Macromol. Mat. Eng. 1993, 211, 121–129. [Google Scholar]
- Zhao, C.H.; Gong, Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M. Self-crosslinked anion exchange membranes by bromination of benzylmethyl-containing poly(sulfone)s for direct methanol fuel cells. Int. J. Hydrogen Energy 2012, 37, 11383–11393. [Google Scholar] [CrossRef]
- Sata, T.; Yamane, Y.; Matsusaki, K. Preparation and properties of anion exchange membranes having pyridinium or pyridinium derivatives as anion exchange groups. J. Polym. Chem. Part A Polym. Chem. 1998, 36, 49–58. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Zhang, S. Novel Hydroxide-Conducting Polyelectrolyte Composed of an Poly(arylene ether sulfone) Containing Pendant Quaternary Guanidinium Groups for Alkaline Fuel Cell Applications. Macromolecules 2010, 43, 3890–3896. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Qu, C. Imidazolium functionalized polysulfone anion exchange membrane for fuel cell application. J. Mater. Chem. 2011, 21, 12744–12752. [Google Scholar] [CrossRef]
- Rebeck, N.T.; Li, Y.; Knauss, D.M. Poly(phenylene oxide) Copolymer Anion Exchange Membranes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1770–1778. [Google Scholar] [CrossRef]
- Hibbs, M.R.; Hickner, M.A.; Alam, T.M.; McIntyre, S.K.; Fujimoto, C.H.; Cornelius, C.J. Transport properties of hydroxide and proton conducting membranes. Chem. Mater. 2008, 20, 2566–2573. [Google Scholar] [CrossRef]
- Poynton, S.D.; Varcoe, J.R. Reduction of the monomer quantities required for the preparation of radiation-grafted alkaline anion-exchange membranes. Solid State Ion. 2015, 277, 38–43. [Google Scholar] [CrossRef]
- Dang, H.S.; Jannasch, P. Exploring Different Cationic Alkyl Side Chain Designs for Enhanced Alkaline Stability and Hydroxide Ion Conductivity of Anion-Exchange Membranes. Macromolecules 2015, 48, 5742–5751. [Google Scholar] [CrossRef]
- Zhu, L.; Pan, Y.; Wang, Y.; Han, J.; Zhuang, L.; Hickner, M.A. Multication Side Chain Anion Exchange Membranes. Macromolecules 2016, 49, 815–824. [Google Scholar] [CrossRef]
- Thomas, O.D.; Soo, K.J.W.Y.; Peckham, T.J.; Kulkarni, M.P.; Holdcroft, S. A Stable Hydroxide-Conducting Polymer. J. Am. Chem. Soc. 2012, 134, 10753–10756. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.G.; Holdcroft, S. Hydroxide-Stable Ionenes. ACS Macro Lett. 2014, 3, 444–447. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Yang, Y.; Brenner, T.M.; Seifert, S.; Yan, Y.; Liberatore, M.W.; Herring, A.M. Anion Transport in a Chemically Stable, Sterically Bulky α-C Modified Imidazolium Functionalized Anion Exchange Membrane. J. Phys. Chem. C 2014, 118, 15136–15145. [Google Scholar] [CrossRef]
- Lin, X.; Varcoe, J.R.; Poynton, S.D.; Liang, X.; Ong, A.L.; Ran, J.; Li, Y.; Xu, T. Alkaline polymer electrolytes containing pendant dimethylimidazolium groups for alkaline membrane fuel cells. J. Mater. Chem. A 2013, 1, 7262–7269. [Google Scholar] [CrossRef]
- Kim, D.S.; Labouriau, A.; Guiver, M.D.; Kim, Y.S. Guanidinium-Functionalized Anion Exchange Polymer Electrolytes via Activated Fluorophenyl-Amine Reaction. Chem. Mater. 2011, 23, 3795–3797. [Google Scholar] [CrossRef]
- Kim, D.S.; Fujimoto, C.H.; Hibbs, M.R.; Laboriau, A.; Choe, Y.K.; Kim, Y.S. Resonance Stabilized Perfluorinated lonomers for Alkaline Membrane Fuel Cells. Macromolecules 2013, 46, 7826–7833. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.H.; Kim, M.K.; Yun, S.H.; Moon, S.H. Stability of composite anion exchange membranes with various functional groups and their performance for energy conversion. J. Memb. Sci. 2013, 443, 28–35. [Google Scholar] [CrossRef]
- Zha, Y.; Disabb-Miller, M.L.; Johnson, Z.D.; Hickner, M.A.; Tew, G.N. Metal-Cation-Based Anion Exchange Membranes. J. Am. Chem. Soc. 2012, 134, 4493–4496. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, L.; Pan, J.; Zhu, Y.; Ge, X.; Yang, Z.; Ran, J.; Xu, T. A mechanically robust anion exchange membrane with high hydroxide conductivity. J. Memb. Sci. 2016, 504, 47–54. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, G.; Zhang, K.; He, G.; Jia, J.; Yu, H.; Gai, F.; Li, L.; Hao, C.; Zhang, F. Guanidimidazole-quaternized and cross-linked alkaline polymer electrolyte membrane for fuel cell application. J. Memb. Sci. 2016, 501, 100–108. [Google Scholar] [CrossRef]
- Katzfuss, A.; Gogel, V.; Jörissen, L.; Kerres, J. The application of covalently cross-linked BrPPO as AEM in alkaline DMFC. J. Memb. Sci. 2013, 425–426, 131–140. [Google Scholar] [CrossRef]
- Katzfuss, A.; Poynton, S.; Varcoe, J.; Gogel, V.; Storr, U.; Kerres, J. Methylated polybenzimidazole and its application as a blend component in covalently cross-linked anion-exchange membranes for DMFC. J. Memb. Sci. 2014, 465, 129–137. [Google Scholar] [CrossRef]
- Morandi, C.G.; Peach, R.; Krieg, H.M.; Kerres, J. Novel morpholinium-functionalized anion-exchange PBI-polymer blends. J. Mater. Chem. A 2015, 3, 1110–1120. [Google Scholar] [CrossRef]
- Kerres, J.; Atanasov, V. Cross-linked PBI-based high-temperature membranes: Stability, conductivity and fuel cell performance. Int. J. Hydrogen Energy 2015, 40, 14723–14735. [Google Scholar] [CrossRef]
- Morandi, C.G.; Peach, R.; Krieg, H.M.; Kerres, J. Novel imidazolium-functionalized anion-exchange polymer PBI blend membranes. J. Memb. Sci. 2015, 476, 256–263. [Google Scholar] [CrossRef]
- Pan, J.; Zhu, L.; Han, J.; Hickner, M.A. Mechanically Tough and Chemically Stable Anion Exchange Membranes from Rigid-Flexible Semi-Interpenetrating Networks. Chem. Mater. 2015, 27, 6689–6698. [Google Scholar] [CrossRef]
- Lu, W.; Shao, Z.-G.; Zhang, G.; Zhao, Y.; Yi, B. Crosslinked poly(vinylbenzyl chloride) with a macromolecular crosslinker for anion exchange membrane fuel cells. J. Power Sources 2014, 248, 905–914. [Google Scholar] [CrossRef]
- Aili, D.; Jankova, K.; Li, Q.; Bjerrum, N.J.; Jensen, J.O. The stability of poly(2,2′-(m-phenylene)-5,5′-bibenzimidazole) membranes in aqueous potassium hydroxide. J. Membr. Sci. 2015, 492, 422–429. [Google Scholar] [CrossRef]
- Aili, D.; Jankova, K.; Han, J.; Bjerrum, N.J.; Jensen, J.O.; Li, Q. Understanding ternary poly(potassium benzimidazolide)-based polymer electrolytes. Polymer 2016, 84, 304–310. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Ma, W.; Zhao, S.; Xu, Z.; Sun, G. Highly stable poly(ethylene glycol)-grafted alkaline anion exchange membranes. J. Mater. Chem. A 2016, 4, 3886–3892. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; Wei, B.; Chen, Y.; Xu, T. Simultaneous Enhancements of Conductivity and Stability for Anion Exchange Membranes (AEMs) through Precise Structure Design. Sci. Rep. 2014, 4, 6486–6490. [Google Scholar] [CrossRef] [PubMed]
- Kerres, J.; Ullrich, A.; Hein, M.; Gogel, V.; Friedrich, K.A.; Jörissen, L. Cross-Linked Polyaryl Blend Membranes for Polymer Electrolyte Fuel Cells. Fuel Cells 2004, 4, 105–112. [Google Scholar] [CrossRef]
- Danks, T.N.; Slade, R.C.T.; Varcoe, J.R. Comparison of PVDF- and FEP-based radiation-grafted alkaline anion-exchange membranes for use in low temperature portable DMFCs. J. Mater. Chem. 2002, 12, 3371–3373. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Q.; Wang, J.; Zhang, S.; Li, S. Synthesis and alkaline stability of novel cardo poly(aryl ether sulfone)s with pendent quaternary ammonium aliphatic side chains for anion exchange membranes. Polymer 2010, 51, 5407–5416. [Google Scholar] [CrossRef]
- Chromik, A.; Kerres, J. Degradation studies on acid-base blends for both LT and intermediate T fuel cells. Solid State Ion. 2013, 252, 140–151. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Liu, P.; Gao, L.; Che, Q.; He, R. Epoxides cross-linked hexafluoropropylidene polybenzimidazole membranes for application as high temperature proton exchange membranes. Electrochim. Acta 2015, 160, 281–287. [Google Scholar] [CrossRef]























| Membrane (No.) | IECcalc (meq OH−/g) | IECexp (meq OH−/g) | IECexp * (meq OH−/g) | Weight Loss ** (%) | σCl− (S/cm) | σCl−* (S/cm) | WU (%, RT) | WU (%, 90 °C) |
|---|---|---|---|---|---|---|---|---|
| 2256_3C_NF | 2.3 | 2.9 | 3.17 | 2.4 | 8.2 | 1.1 | 49 | 51 |
| 2257_3C_NF | 2.3 | 0.39 | 0.45 | 7.3 | 0.17 | 0.12 | 7 | 8 |
| 2179B_3C_NF | 2.3 | 2.5 | 2.64 | 5.8 | 10.7 | 15.9 | 63 | 100 |
| 2223_3C_NF | 2.0 | 2.97 | 3.15 | 2.5 | 10.8 | 5.4 | 56 | 79 |
| 2177_4C_NF | 2.3 | 2.2 | 2.44 | 3.4 | 14.0 | 10.6 | 36 | 42 |
| 2175_4C_NF | 2.3 | 2.92 | 2.96 | 2.4/0 § | 29.3 | 72.7 | 367 | n.m. |
| 2176_4C_NF | 2.3 | 2.79 | 2.84 | 2.6/3 § | 21.6 | 69.9 | 370 | 436 |
| 2190A_4C_NF | 2.0 | 2.1 | 2.73 | 4.1 | 14.3 | 16.3 | 67 | 120 |
| 2261_4C_NF | 2.3 | 2.2 | n.a. | 0.6 | 42.8 | 32.2 | 132 | 212 |
| 2262_4C_NF | 2.6 | n.a. | 3.53 | 2.1 | 72.9 | 56.9 | 242 | 377 |
| 2264_4C_NF | 2.0 | 3.07 | 2.93 | 0 | 14.0 | 11.1 | 71 | 90 |
| 2265_4C_NF | 1.7 | 3.07 | 3.04 | 0 | 4.9 | 1.3 | 41 | 47 |
| 2279_4C_NF | 1.4 | 2.89 | 3.04 | 0 | 0.97 | 0.1 | 28 | 28 |
| 2267_4C_NF | 2.3 | 2.92 | 2.84 | 0 | 51.7 | 60.5 | 290 | 371 |
| 2246_4C_F | 2.0 | 2.23 | 2.38 *** | 2.1 | 3.9 | 4.4 *** | 29 | 34 |
| 2258_4C_F | 2.3 | 2.42 | 2.53 | 1.9 | 26.4 | 16.1 | 77 | 91 |
| 2259_4C_F | 2.6 | 2.41 | 2.74 | 0.9 | 45.4 | 46.8 | 157 | 258 |
| Membrane Type * (No.) | IECcalc (meq OH−/g) | Type amine | PVBCl-AEM (%) | PBI (%) | S-Polymer (%) | Type PEG (Da) | PEG (%) |
|---|---|---|---|---|---|---|---|
| 2256_3C_NF | 2.3 | NMM | 58.3 | PBIOO/32.8 | SPPSU **/8.9 | - | - |
| 2257_3C_NF | 2.3 | PMP | 70.0 | PBIOO/23.6 | SPPSU/6.4 | - | - |
| 2179B_3C_NF | 2.3 | TMIm | 63.5 | PBIOO/28.7 | SPPSU/7.8 | - | - |
| 2223_3C_NF | 2.0 | TMIm | 54.9 | PBIOO/38.4 | SPPSU/6.8 | - | - |
| 2177_4C_NF | 2.3 | TMIm | 62.9 | PBIOO/26.0 | SPPSU/7.4 | 6000 | 3.7 |
| 2175_4C_NF | 2.3 | TMIm | 63.5 | PBIOO/20.9 | SPPSU/7.8 | 500 | 7.8 |
| 2176_4C_NF | 2.3 | TMIm | 63.5 | PBIOO/20.9 | SPPSU/7.8 | 6000 | 7.8 |
| 2190A_4C_NF | 2.0 | TMIm | 54.9 | PBIOO/27.9 | SPPSU/6.8 | 6000 | 10.4 |
| 2261_4C_NF | 2.3 | TMIm | 62.9 | PBIOO/24.1 | SPPSU/7.4 | 500 | 5.6 |
| 2262_4C_NF | 2.6 | TMIm | 69.9 | PBIOO/19.5 | SPPSU/6.0 | 500 | 4.5 |
| 2264_4C_NF | 2.0 | TMIm | 55.9 | PBIOO/28.6 | SPPSU/8.8 | 500 | 6.6 |
| 2265_4C_NF | 1.7 | TMIm | 48.7 | PBIOO/33.4 | SPPSU/10.3 | 500 | 7.7 |
| 2279_4C_NF | 1.4 | TMIm | 41.6 | PBIOO/41.6 | SPPSU/11.7 | 500 | 8.8 |
| 2267_4C_NF | 2.3 | TMIm | 65.2 | PBIOO/18.2 | SPPSU/12.3 | 500 | 4.2 |
| 2246_4C_F | 2.0 | TMIm | 56.6 | F6PBI/28.98 | SFPE ***/7.8 | 500 | 6.7 |
| 2258_4C_F | 2.3 | TMIm | 63.5 | F6PBI/24.4 | SFPE/6.5 | 500 | 5.6 |
| 2259_4C_F | 2.6 | TMIm | 70.5 | F6PBI/19.7 | SFPE/5.3 | 500 | 4.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerres, J.A.; Krieg, H.M. Poly(vinylbenzylchloride) Based Anion-Exchange Blend Membranes (AEBMs): Influence of PEG Additive on Conductivity and Stability. Membranes 2017, 7, 32. https://doi.org/10.3390/membranes7020032
Kerres JA, Krieg HM. Poly(vinylbenzylchloride) Based Anion-Exchange Blend Membranes (AEBMs): Influence of PEG Additive on Conductivity and Stability. Membranes. 2017; 7(2):32. https://doi.org/10.3390/membranes7020032
Chicago/Turabian StyleKerres, Jochen A., and Henning M. Krieg. 2017. "Poly(vinylbenzylchloride) Based Anion-Exchange Blend Membranes (AEBMs): Influence of PEG Additive on Conductivity and Stability" Membranes 7, no. 2: 32. https://doi.org/10.3390/membranes7020032
APA StyleKerres, J. A., & Krieg, H. M. (2017). Poly(vinylbenzylchloride) Based Anion-Exchange Blend Membranes (AEBMs): Influence of PEG Additive on Conductivity and Stability. Membranes, 7(2), 32. https://doi.org/10.3390/membranes7020032