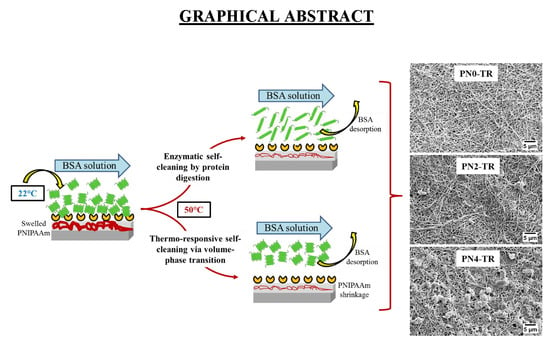
Dual Functional Ultrafiltration Membranes with Enzymatic Digestion and Thermo-Responsivity for Protein Self-Cleaning
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PVDF/nylon-6,6/PNIPAAm Membrane
2.3. Preparation of Biocatalytic PVDF/nylon-6,6/PNIPAAm Membranes
2.4. Membrane Characterization
2.5. Quantification of Immobilized TR and Its Activity against BSA
2.6. Fouling Studies
2.7. Storage Studies and Effect of Thermo-Responsivity on Enzyme Stability
3. Results and Discussion
3.1. Enzyme Distribution on Membrane Surface
3.2. Surface Density of Immobilized Enzyme
3.3. Membrane Characterization
3.4. Enzyme Activity Evaluation Across the Nano-Composite Membranes
3.5. Protein Fouling Studies
3.6. Storage Studies & Effect of Thermo-Responsivity on Enzyme Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PVDF | poly(vinylidene fluoride) |
| PNIPAAm | poly(N-isopropylacrylamide) |
| BSA | bovine serum albumin |
| NaCl | sodium chloride |
| CaCl2 | calcium chloride |
| UF | ultrafiltration |
| PET | polyethylene terephthalate |
| PLA | poly(lactic acid) |
| LCST | lower critical solution temperature |
| TiO2 | titanium dioxide |
| ZrO2 | zirconium dioxide |
| TR | trypsin |
| PVP | poly(vinyl pyrrolidone) |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| NHS | N-hydroxysuccinimide |
| DMAC | N,N′-dimethylacetamide |
| TCA | trichloroacetic acid |
| DI | deionised |
| SEM | scanning electron microscopy |
| CAw | water contact angle |
| DU | digestion unit |
| RPD | rate of permeance decline |
| PRR | permeance recovery rate |
References
- Shi, Q.; Su, Y.; Ning, X.; Chen, W.; Peng, J.; Jiang, Z. Trypsin-enabled construction of anti-fouling and self-cleaning polyethersulfone membrane. Bioresour. Technol. 2011, 102, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Jim, K.J.; Fane, A.G.; Fell, C.J.D.; Joy, D.C. fouling mechanisms of membranes during protein ultrafiltration. J. Membr. Sci. 1992, 68, 79–91. [Google Scholar] [CrossRef]
- Ho, C.-C.; Zydney, A.L. A combined pore blockage and cake filtration model for protein fouling during microfiltration. J. Colloid Interface Sci. 2000, 232, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zheng, J.; Wang, M.; Zhang, H.; Han, C.C. High performance ultrafiltration membrane based on modified chitosan coating and electrospun nanofibrous pvdf scaffolds. J. Membr. Sci. 2012, 394, 209–217. [Google Scholar] [CrossRef]
- Vanangamudi, A.; Dumée, L.F.; Duke, M.C.; Yang, X. Nanofiber composite membrane with intrinsic janus surface for reversed-protein-fouling ultrafiltration. ACS Appl. Mater. Interfaces 2017, 9, 18328–18337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-P.; Yi, Z.; Liu, F.; Wei, X.-Z.; Zhu, B.-K.; Xu, Y.-Y. Amphiphilic graft copolymers based on ultrahigh molecular weight poly(styrene-alt-maleic anhydride) with poly(ethylene glycol) side chains for surface modification of polyethersulfone membranes. Eur. Polym. J. 2008, 44, 1907–1914. [Google Scholar] [CrossRef]
- Zhao, X.; He, C. Efficient preparation of super antifouling pvdf ultrafiltration membrane with one step fabricated zwitterionic surface. ACS Appl. Mater. Interfaces 2015, 7, 17947–17953. [Google Scholar] [CrossRef] [PubMed]
- Prince, J.A.; Bhuvana, S.; Anbharasi, V.; Ayyanar, N.; Boodhoo, K.V.K.; Singh, G. Self-cleaning metal organic framework (mof) based ultra filtration membranes—A solution to bio-fouling in membrane separation processes. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Vanangamudi, A.; Saeki, D.; Dumée, L.F.; Duke, M.; Vasiljevic, T.; Matsuyama, H.; Yang, X. Surface-engineered biocatalytic composite membranes for reduced protein fouling and self-cleaning. ACS Appl. Mater. Interfaces 2018, 10, 27477–27487. [Google Scholar] [CrossRef] [PubMed]
- Chede, S.; Escobar, I.C. Fouling control using temperature responsive n-isopropylacrylamide (nipaam) membranes. Environ. Prog. Sustain. Energy 2016, 35, 416–427. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Velicangil, O.; Howell, J.A. Self-cleaning membranes for ultrafiltration. Biotechnol. Bioeng. 1981, 23, 843–854. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Werner, C. Enzymes for antifouling strategies. J. Adhes. Sci. Technol. 2011, 25, 2317–2344. [Google Scholar] [CrossRef]
- Sun, L.; Liang, H.; Yuan, Q.; Wang, T.; Zhang, H. Study on a carboxyl-activated carrier and its properties for papain immobilization. J. Chem. Technol. Biotechnol. 2012, 87, 1083–1088. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Grate, J.W.; Wang, P. Nanostructures for enzyme stabilization. Chem. Eng. Sci. 2006, 61, 1017–1026. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Wan, L.-S.; Liu, Z.-M.; Huang, X.-J.; Xu, Z.-K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Al-Attabi, R.; Morsi, Y.; Schütz, J.A.; Dumée, L.F. One-pot synthesis of catalytic molybdenum based nanocomposite nano-fiber membranes for aerosol air remediation. Sci. Total Environ. 2019, 647, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Al-Attabi, R.; Dumée, L.F.; Schütz, J.A.; Morsi, Y. Pore engineering towards highly efficient electrospun nanofibrous membranes for aerosol particle removal. Sci. Total Environ. 2018, 625, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Vanangamudi, A.; Yang, X.; Duke, M.C.; Dumee, L.F. Nanofibers for membrane applications. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Jia, H.; Zhu, G.; Vugrinovich, B.; Kataphinan, W.; Reneker, D.H.; Wang, P. Enzyme-carrying polymeric nanofibers prepared via electrospinning for use as unique biocatalysts. Biotechnol. Prog. 2002, 18, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Park, T.G. Surface functionalized electrospun biodegradable nanofibers for immobilization of bioactive molecules. Biotechnol. Prog. 2006, 22, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Srbová, J.; Slováková, M.; Křípalová, Z.; Žárská, M.; Špačková, M.; Stránská, D.; Bílková, Z. Covalent biofunctionalization of chitosan nanofibers with trypsin for high enzyme stability. React. Funct. Polym. 2016, 104, 38–44. [Google Scholar] [CrossRef]
- Silva, T.R.; Rodrigues, D.P.; Rocha, J.M.S.; Gil, M.H.; Pinto, S.C.S.; Lopes-da-Silva, J.A.; Guiomar, A.J. Immobilization of trypsin onto poly(ethylene terephthalate)/poly(lactic acid) nonwoven nanofiber mats. Biochem. Eng. J. 2015, 104, 48–56. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Schulze, A.; Breite, D.; Kim, Y.; Schmidt, M.; Thomas, I.; Went, M.; Fischer, K.; Prager, A. Bio-inspired polymer membrane surface cleaning. Polymers 2017, 9, 97. [Google Scholar] [CrossRef]
- Schulze, A.; Stoelzer, A.; Striegler, K.; Starke, S.; Prager, A. Biocatalytic self-cleaning polymer membranes. Polymers 2015, 7, 1837–1849. [Google Scholar] [CrossRef]
- Moreno-Cortez, I.E.; Romero-García, J.; González-González, V.; García-Gutierrez, D.I.; Garza-Navarro, M.A.; Cruz-Silva, R. Encapsulation and immobilization of papain in electrospun nanofibrous membranes of pva cross-linked with glutaraldehyde vapor. Mater. Sci. Eng. C 2015, 52, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.P.; Dubey, N.; Simon, F.; Stamm, M. Thermo responsive ultrafiltration membranes of grafted poly(n-isopropyl acrylamide) via polydopamine. RSC Adv. 2014, 4, 34073–34083. [Google Scholar] [CrossRef]
- Bae, Y.H.; Okano, T.; Kim, S.W. Temperature dependence of swelling of crosslinked poly(n,n′-alkyl substituted acrylamides) in water. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 923–936. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, L.; Ma, R.; Zhang, W.; An, Y.; Zhu, X.X. Synthesis and micellization of thermo- and ph-responsive block copolymer of poly(n-isopropylacrylamide)-block-poly(4-vinylpyridine). Polymer 2007, 48, 1711–1717. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.-H.; Yan, B.-H.; Wu, D.; Zhang, Q.-Q. Thermo-responsive and antifouling PVDF nanocomposited membranes based on PNIPAAm modified TiO2 nanoparticles. Chin. J. Polym. Sci. 2014, 32, 892–905. [Google Scholar] [CrossRef]
- Guo, H.; Huang, J.; Wang, X. The alternate temperature-change cleaning behaviors of pnipaam grafted porous polyethylene membrane fouled by proteins. Desalination 2008, 234, 42–50. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, A.; Zhang, Y.; Li, M.; Wang, J.; Zhao, Y.; Xing, W. Fabrication of temperature-responsive zro2 tubular membranes, grafted with poly (n-isopropylacrylamide) brush chains, for protein removal and easy cleaning. J. Membr. Sci. 2014, 450, 351–361. [Google Scholar] [CrossRef]
- Available online: http://www.worthington-biochem.com/TRY/default.html (accessed on 18 September 2018).
- Khaldi, M.; Blanpain-Avet, P.; Guérin, R.; Ronse, G.; Bouvier, L.; André, C.; Bornaz, S.; Croguennec, T.; Jeantet, R.; Delaplace, G. Effect of calcium content and flow regime on whey protein fouling and cleaning in a plate heat exchanger. J. Food Eng. 2015, 147, 68–78. [Google Scholar] [CrossRef]
- Jia, F.; Narasimhan, B.; Mallapragada, S. Materials-based strategies for multi-enzyme immobilization and co-localization: A review. Biotechnol. Bioeng. 2014, 111, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Z.; Zhu, L.-P.; Zhu, B.-K.; Xu, Y.-Y. Poly( n-isopropylacrylamide) grafted poly(vinylidene fluoride) copolymers for temperature-sensitive membranes. J. Membr. Sci. 2011, 366, 176–183. [Google Scholar] [CrossRef]
- Kang, K.; Kan, C.; Yeung, A.; Liu, D. The immobilization of trypsin on soap-free p(mma-ea-aa) latex particles. Mater. Sci. Eng. C 2006, 26, 664–669. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, S.; Tang, X.; Kaiser, N.K.; Bruce, J.E. A bifunctional monolithic column for combined protein preconcentration and digestion for high throughput proteomics research. J. Chromatogr. B 2007, 849, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Ohta, M.; Sugiyama, N.; Oshima, K.; Yamauchi, T.; Miyauchi, S. Properties of α-chymotrypsin covalently immobilized on poly(acrylic acid)-grafted magnetite particles. Polym. J. 1999, 31, 274–278. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanangamudi, A.; Dumée, L.F.; Duke, M.C.; Yang, X. Dual Functional Ultrafiltration Membranes with Enzymatic Digestion and Thermo-Responsivity for Protein Self-Cleaning. Membranes 2018, 8, 85. https://doi.org/10.3390/membranes8030085
Vanangamudi A, Dumée LF, Duke MC, Yang X. Dual Functional Ultrafiltration Membranes with Enzymatic Digestion and Thermo-Responsivity for Protein Self-Cleaning. Membranes. 2018; 8(3):85. https://doi.org/10.3390/membranes8030085
Chicago/Turabian StyleVanangamudi, Anbharasi, Ludovic F. Dumée, Mikel C. Duke, and Xing Yang. 2018. "Dual Functional Ultrafiltration Membranes with Enzymatic Digestion and Thermo-Responsivity for Protein Self-Cleaning" Membranes 8, no. 3: 85. https://doi.org/10.3390/membranes8030085
APA StyleVanangamudi, A., Dumée, L. F., Duke, M. C., & Yang, X. (2018). Dual Functional Ultrafiltration Membranes with Enzymatic Digestion and Thermo-Responsivity for Protein Self-Cleaning. Membranes, 8(3), 85. https://doi.org/10.3390/membranes8030085