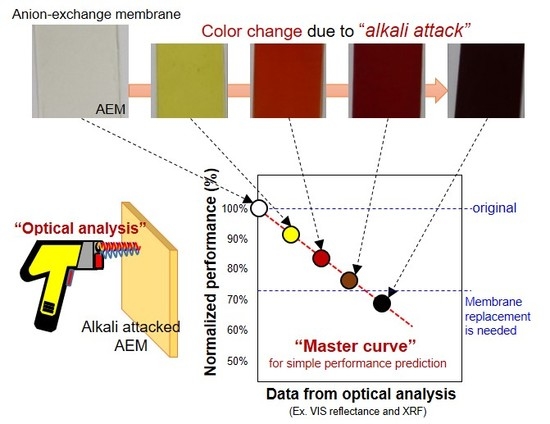
Alkali Attack on Anion Exchange Membranes with PVC Backing and Binder: II Prediction of Electrical and Mechanical Performances from Simple Optical Analyses
Abstract
:1. Introduction
2. Experimental
2.1. Membranes and Chemicals
2.2. Alkali Immersion Test
2.3. Membrane Characteristics and Performance after Alkali Immersion
2.4. Optical Analyses for Alkali Immersed AMXs
2.4.1. VIS Reflectance Measurement
2.4.2. XRF Measurement
3. Results and Discussion
3.1. Color Change of the Alkali Attacked AMX
3.2. VIS Reflectance Results
3.2.1. Time Course under Different Immersion Conditions
3.2.2. Correlation to the Performance of the Alkali-Attacked AMXs
3.3. Handheld XRF Results
3.3.1. Time Course under Different Immersion Conditions
3.3.2. Correlation to the Performance of the Alkali Attacked AMXs
3.4. Comparison between VIS Reflectance and Handheld XRF Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A1. Relationship between Normalized VIS Reflectance and Resulting Performance of the AMX after Alkali Attacking

Appendix A2. Relationship between Normalized Cl Intensity and Resulting Performance of the AMX after Alkali Attacking

References
- Strathmann, H. Ion-Exchange Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Tanaka, Y. Ion Exchange Membranes Fundamentals and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Nie, X.-Y.; Sun, S.-Y.; Song, X.; Yu, J.-G. Further investigation into lithium recovery from salt lake brines with different feed characteristics by electrodialysis. J. Membr. Sci. 2017, 530, 185–191. [Google Scholar] [CrossRef]
- Peraki, M.; Ghazanfari, E.; Pinder, G.F.; Harrington, T.L. Electrodialysis: An application for the environmental protection in shale-gas extraction. Sep. Purif. Technol. 2016, 161, 96–103. [Google Scholar] [CrossRef]
- Taniguchi, I.; Yamada, T. Low energy CO2 capture by electrodialysis. Energy Procedia 2017, 114, 1615–1620. [Google Scholar] [CrossRef]
- Dara, S.; Lindstrom, M.; English, J.; Bonakdarpour, A.; Wetton, B.; Wilkinson, D.P. Conversion of saline water and dissolved carbon dioxide into value-added chemicals by electrodialysis. J. CO2 Util. 2017, 19, 177–184. [Google Scholar] [CrossRef]
- Sata, T. Ion Exchange Membranes: Preparation, Characterization, Modification and Application; The Royal Society of Chemistry: Cambridge, UK, 2004. [Google Scholar]
- Garcia-Cesquez, W.; Ghalloussi, R.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Structure and properties of heterogeneous and homogeneous ion-exchange membranes subjected to ageing in sodium hypochlorite. J. Membr. Sci. 2014, 452, 104–116. [Google Scholar] [CrossRef]
- Tanaka, N.; Higa, M. Organic fouling properties of anion-exchange membranes with various electrodialysis conditions. Bull. Soc. Sea Water Sci. Jpn. 2011, 65, 362–363. [Google Scholar] [CrossRef]
- Choi, J.-H.; Moon, S.-H. Structural change of ion-exchange membrane surfaces under high electric fields and its effects on membrane properties. J. Colloid Interface Sci. 2003, 265, 93–100. [Google Scholar] [CrossRef]
- Sata, T.; Tsujimoto, M.; Yamaguchi, T.; Matsusaki, K. Change of anion exchange membranes in an aqueous sodium hydroxide solution at high temperature. J. Membr. Sci. 1996, 112, 161–170. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Pismenskaya, N.; Grande, D. Evolution of anion-exchange membrane properties in a full scale electrodialysis stack. J. Membr. Sci. 2013, 446, 255–265. [Google Scholar] [CrossRef]
- Doi, S.; Yasukawa, H.; Kakihana, Y.; Higa, M. Alkali attack on anion exchange membranes with PVC backing and binder: Effect on performance and correlation between them. J. Membr. Sci. 2019, 573, 85–96. [Google Scholar] [CrossRef]
- Tanaka, N.; Nagase, M.; Higa, M. Preparation of aliphatic-hydrocarbon-based anion-exchange membranes and their anti-organic-fouling properties. J. Membr. Sci. 2011, 384, 27–36. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Effects of acid-base cleaning procedure on structure and properties of anion-exchange membranes used in Electrodialysis. J. Membr. Sci. 2016, 507, 12–23. [Google Scholar] [CrossRef]







| Methods | Used IEMs | Measurable | Possibility as DIP | Reference | |
|---|---|---|---|---|---|
| UV spectroscopy | Absorbance | Neosepta® AMX | Amount of polyene formation due to dehydrochlorination from PVC | No | [15] |
| Transmittance | - | No | - | ||
| Reflectance (relative total) | Neosepta® AMX | Yes | In this study | ||
| X-ray fluorescence | Cl intensity (2.6 keV, Ka1) | Neosepta® AMX | Total Cl amount within the membrane | Yes | In this study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doi, S.; Kinoshita, M.; Yasukawa, M.; Higa, M. Alkali Attack on Anion Exchange Membranes with PVC Backing and Binder: II Prediction of Electrical and Mechanical Performances from Simple Optical Analyses. Membranes 2018, 8, 133. https://doi.org/10.3390/membranes8040133
Doi S, Kinoshita M, Yasukawa M, Higa M. Alkali Attack on Anion Exchange Membranes with PVC Backing and Binder: II Prediction of Electrical and Mechanical Performances from Simple Optical Analyses. Membranes. 2018; 8(4):133. https://doi.org/10.3390/membranes8040133
Chicago/Turabian StyleDoi, Shoichi, Maki Kinoshita, Masahiro Yasukawa, and Mitsuru Higa. 2018. "Alkali Attack on Anion Exchange Membranes with PVC Backing and Binder: II Prediction of Electrical and Mechanical Performances from Simple Optical Analyses" Membranes 8, no. 4: 133. https://doi.org/10.3390/membranes8040133
APA StyleDoi, S., Kinoshita, M., Yasukawa, M., & Higa, M. (2018). Alkali Attack on Anion Exchange Membranes with PVC Backing and Binder: II Prediction of Electrical and Mechanical Performances from Simple Optical Analyses. Membranes, 8(4), 133. https://doi.org/10.3390/membranes8040133