Perfluorosulfonic Acid Membranes Thermally Treated and Modified by Dopants with Proton-Acceptor Properties for Asparaginate and Potassium Ions Determination in Pharmaceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents Used
2.2. Membrane Preparation
2.2.1. Preparation of Hybrid Membranes by Casting
2.2.2. Preparation of Hybrid Membranes In Situ
2.2.3. Membrane Treatment
2.3. Membrane Regeneration
2.4. Apparatus and Procedures
3. Results and Discussion
3.1. Properties of Membranes
3.2. Characteristics of DP-Sensors and Multisensory Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prasad, B.B.; Srivastava, A.; Tiwari, M.P. Molecularly imprinted polymer-matrix nanocomposite for enantioselective electrochemical sensing of d-and l-aspartic acid. Mater. Sci. Eng. C 2013, 33, 4071–4080. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Jaiswal, S.; Singh, K. Ultra-trace analysis of d-and l-aspartic acid applying one-by-one approach on a dual imprinted electrochemical sensor. Sens. Actuator B-Chem. 2017, 240, 631–639. [Google Scholar] [CrossRef]
- Heli, H.; Hajjizadeh, M.; Jabbari, A.; Moosavi-Movahedi, A.A. Fine steps of electrocatalytic oxidation and sensitive detection of some amino acids on copper nanoparticles. Anal. Biochem. 2009, 388, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Röhlen, D.L.; Pilas, J.; Schöning, M.J.; Selmer, T. Development of an amperometric biosensor platform for the combined determination of L-malic, fumaric, and L-aspartic acid. Appl. Biochem. Biotechnol. 2017, 183, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Guha, S.; Banerjee, A.; Lohar, S.; Sahana, A.; Das, D. 2-(2-Pyridyl) benzimidazole based Co (II) complex as an efficient fluorescent probe for trace level determination of aspartic and glutamic acid in aqueous solution: A displacement approach. Org. Biomol. Chem. 2011, 9, 7097–7104. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef]
- Sidel’nikov, A.V.; Zil’berg, R.A.; Yarkaeva, Y.A.; Maistrenko, V.N.; Kraikin, V.A. Voltammetric identification of antiarrhythmic medicines using principal component analysis. J. Anal. Chem. 2015, 70, 1261–1266. [Google Scholar] [CrossRef]
- Kulapina, E.G.; Snesarev, S.V.; Makarova, N.M.; Pogorelova, E.S. Potentiometric sensor arrays for the individual determination of penicillin class antibiotics using artificial neural networks. J. Anal. Chem. 2011, 66, 78–83. [Google Scholar] [CrossRef]
- Wesoły, M.; Cetó, X.; Del Valle, M.; Ciosek, P.; Wróblewski, W. Quantitative analysis of active pharmaceutical ingredients (APIs) using a potentiometric electronic tongue in a SIA flow system. Electroanalysis 2016, 28, 626–632. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lopa, N.S.; Kim, K.; Lee, J.J. Selective detection of L-tyrosine in the presence of ascorbic acid, dopamine, and uric acid at poly (thionine)-modified glassy carbon electrode. J. Electroanal. Chem. 2015, 754, 87–93. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Dadkhah-Tehrani, S.; Karimi-Maleh, H. A voltammetric sensor for the simultaneous determination of L-cysteine and tryptophan using a p-aminophenol-multiwall carbon nanotube paste electrode. Anal. Sci. 2011, 27, 409–414. [Google Scholar] [CrossRef]
- Liang, R.; Yin, T.; Qin, W. A simple approach for fabricating solid-contact ion-selective electrodes using nanomaterials as transducers. Anal. Chim. Acta 2015, 853, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Scagion, V.P.; Pavinatto, A.; Sanfelice, R.C.; Mattoso, L.H.; Correa, D.S. Electronic tongue based on nanostructured hybrid films of gold nanoparticles and phthalocyanines for milk analysis. J. Nanomater. 2015, 16, 402–409. [Google Scholar] [CrossRef]
- Facure, M.H.; Mercante, L.A.; Mattoso, L.H.; Correa, D.S. Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta 2017, 167, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, K.B.R.; Shimizu, F.M.; Scagion, V.P.; Correa, D.S. Ternary nanocomposites based on cellulose nanowhiskers, silver nanoparticles and electrospun nanofibers: Use inan electronic tongue for heavy metal detection. Sens. Actuators B 2019, 290, 387–395. [Google Scholar] [CrossRef]
- Apel, P.Y.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of Membrane Science Development. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Ipatov, A. Recent trends in potentiometric sensor arrays—A review. Anal. Chim. Acta 2010, 678, 149–159. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.C. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Bobreshova, O.V.; Parshina, A.V.; Polumestnaya, K.A.; Safronova, E.Y.; Yankina, K.Y.; Yaroslavtsev, A.B. Perfluorinated sulfocation-exchange membranes modified with zirconia for sensors susceptible to organic anions in multiionic aqueous solutions. Mendeleev Commun. 2012, 2, 83–84. [Google Scholar] [CrossRef]
- Parshina, A.V.; Denisova, T.S.; Safronova, E.Y.; Karavanova, Y.A.; Safronov, D.V.; Bobreshova, O.V.; Yaroslavtsev, A.B. Determination of sulfur-containing anions in alkaline solutions using arrays of DP-sensors based on hybrid perfluorinated membranes with proton-donor dopants. J. Anal. Chem. 2017, 72, 1243–1250. [Google Scholar] [CrossRef]
- Safronova, E.; Parshina, A.; Kolganova, T.; Bobreshova, O.; Pourcelly, G.; Yaroslavtsev, A. Potentiometric sensors arrays based on perfluorinated membranes and silica nanoparticles with surface modified by proton-acceptor groups, for the determination of aspartic and glutamic amino acids anions and potassium cations. J. Electroanal. Chem. 2018, 816, 21–29. [Google Scholar] [CrossRef]
- Berezina, N.P.; Timofeev, S.V.; Kononenko, N.A. Effect of conditioning techniques of perfluorinated sulphocationic membranes on their hydrophylic and electrotransport properties. J. Membr. Sci. 2002, 209, 509–518. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R. Evolution of permanent deformations (or memory) in Nafion 117 membranes with changes in temperature, relative humidity and time, and its importance in the development of medium temperature PEMFCs. Fuel Cells 2009, 9, 410–420. [Google Scholar] [CrossRef]
- Safronova, E.; Safronov, D.; Lysova, A.; Parshina, A.; Bobreshova, O.; Pourcelly, G.; Yaroslavtsev, A. Sensitivity of potentiometric sensors based on Nafion®-type membranes and effect of the membranes mechanical, thermal, and hydrothermal treatments on the on their properties. Sens. Actuator B-Chem. 2017, 240, 1016–1023. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Stenina, I.A.; Yaroslavtsev, A.B. The possibility of changing the transport properties of ion-exchange membranes by their treatment. Pet. Chem. 2017, 57, 299–305. [Google Scholar] [CrossRef]
- Liu, D.; Hickner, M.A.; Case, S.W.; Lesko, J.J. Relaxation of proton conductivity and stress in proton exchange membranes under strain. J. Eng. Mater. Technol. 2006, 128, 503–508. [Google Scholar] [CrossRef]
- DeBonis, D.; Mayer, M.; Omosebi, A.; Besser, R.S. Analysis of mechanism of Nafion® conductivity change due to hot pressing treatment. Renew. Energy 2016, 89, 200–206. [Google Scholar] [CrossRef]
- Parshina, A.V.; Safronova, E.Y.; Titova, T.S.; Safronov, D.V.; Lysova, A.A.; Bobreshova, O.V.; Yaroslavtsev, A.B. Potentiometric Cross-Sensitive Sensors Based on Perfluorinated Membranes Treated at Different Relative Humidity for Codetermination of Cations and Anions in Alkaline Solutions of Amino Acids. Russ. J. Electrochem. 2017, 53, 1294–1299. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Yaroslavtsev, A.B. Relationship between properties of hybrid ion-exchange membranes and dopant nature. Solid State Ion. 2013, 251, 23–27. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Il’in, A.B.; Lysova, A.A.; Yaroslavtsev, A.B. Effect of surface modification with carbon-containing groups on the size, properties, and morphology of silica particles. Inorg. Mater. 2012, 48, 368–373. [Google Scholar] [CrossRef]
- Mikheev, A.G.; Safronova, E.Y.; Yurkov, G.Y.; Yaroslavtsev, A.B. Hybrid materials based on MF-4SC perfluorinated sulfo cation-exchange membranes and silica with proton-acceptor properties. Mend. Comm. 2013, 23, 66–68. [Google Scholar] [CrossRef]








| Object | Method | Sensor Composition | c, mg/mL; c, M | cmin, mg/mL; cmin, M | Accuracy, % | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| Astymin Hepa | Differential pulse anode inversion voltammetry | Graphite electrode/TiO2 nanoparticles, MWCNTs/molecularly imprinted polymer membrane | (12.46–515.53) × 10−6; (0.09361–3.8733) × 10−6 | 1.73 × 10−6; 0.0130 × 10−6 | 96.1–101.3 | A decrease in the selectivity upon reaching imax, a decrease in the response on 15% after 1 month of use, lifetime ~100 measurements | [1] |
| Blood serum | (9.98–524.25) × 10−6; (0.0750–3.9388) × 10−6 | 1.77 × 10−6; 0.0133 × 10−6 | 97.8–102.6 | ||||
| Cerebro-spinal fluid * | (9.98–532.72) × 10−6; (0.0750–4.0024) × 10−6 | 1.79 × 10−6; 0.0134 × 10−6 | 98.0–101.1 | ||||
| Astymin Hepa * | Graphite electrode/Au nanoparticles, MWCNTs/molecularly imprinted polymer membrane | (4.14–68.21) × 10−6; (0.0311–0.5125) × 10−6 | 1.16 × 10−6; 0.00872 × 10−6 | 99–102 | A decrease in the response on 2.68%–2.71% after 3 weeks of use | [2] | |
| Blood serum * | (4.30–69.38) × 10−6; (0.0323–0.5213) × 10−6 | 1.25 × 10−6; 0.00939 × 10−6 | 99–101 | ||||
| Cerebro-spinal fluid * | (4.12–69.27) × 10−6; (0.0310–0.5204) × 10−6 | 1.17 × 10−6; 0.00879 × 10−6 | 99–102 | ||||
| Model solution | Amperometry | Platinum electrode/malate-specific dehydrogenase, diaphorase | 0.1331–1.331; 0.0010–0.0100 | 9.05; 0.068 × 10−3 | - | The complexity of a stable enzyme layer formation, the influence of pH on sensitivity | [3] |
| Model solution | Cyclic voltammetry | Carbon paste electrode/Cu nanoparticles | (39.93–93.17) × 10−3; (0.300–0.700) × 10−3 | 3.993 × 10−3; 0.030 × 10−3 | - | - | [4] |
| Model solution | Fluorimetry | Co (II)+2-(2-pyridyl) benzimidazole complex | 1.331 × 10−3; 1.0 × 10−5 | - | - | - | [5] |
| Treatment Conditions | Composition | ω(H2O), % | P × 108, cm2/s |
|---|---|---|---|
| Without treatment | MF-4SC | 6.5 | 5.62 |
| Nafion | 8.1 | 2.7 | |
| Nafion + SiO2(R1) | 5.1 | 0.21 | |
| Nafion + SiO2(R2) | 4.6 | 0.058 | |
| RH = 60%, t = 95 °C | MF-4SC | 3.1 | 0.0067 |
| Nafion | 4.6 | 0.12 | |
| Nafion + SiO2(R1) | 3.6 | 0.036 | |
| Nafion + SiO2(R2) | 3.6 | 0.052 | |
| tht = 120 °C | MF-4SC | 10.4 | 16.2 |
| Nafion | 9.9 | 32 | |
| Nafion + SiO2(R1) | 3.5 | 0.062 | |
| Nafion + SiO2(R2) | 3.1 | 0.034 | |
| Mechanical deformation of 80%, t = 80 °C | MF-4SC | 5.9 | - * |
| Nafion | 6.7 | - * |
| Composition | ω(H2O), % | P × 108, cm2/s |
|---|---|---|
| MF-4SC | 18.1 | 53 |
| MF-4SC + 3 wt.% SiO2 (5 mol.% R1) | 18.5 | 47 |
| MF-4SC + 3 wt.% SiO2 (10 mol.% R1) | 13.7 | 270 |
| MF-4SC + 3 wt.% SiO2 (5 mol.% R2) | 12.6 | 22 |
| MF-4SC + 3 wt.% SiO2 (10 mol.% R2) | 10.5 | 130 |
| Array | I | II | III | |||
|---|---|---|---|---|---|---|
| DP-sensor composition | Nafion RH = 60% t = 95 °C | Nafion 1.1–1.3 wt. % SiO2(R2) RH = 60 %, t = 95 °C | MF-4SC 3 wt.% SiO2 10 mol.% R1 | MF-4SC 3 wt.% SiO2 10 mol.% R2 | Nafion | MF-4SC tht = 120 °C |
| τresp, min | <1 | |||||
| Drift, mV/h | insignificant | insignificant | 3 ± 1.5 | 5 ± 2 | 7 ± 5 | insignificant |
| s2, mV2 | 50 | 30 | 1.6 | 9 | 60 | 50 |
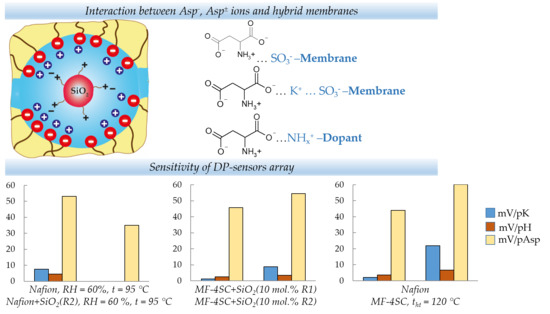
| b1, mV/pK | 7.6 ± 0.8 | insignificant | 1.2 ± 0.6 | 8.8 ± 0.4 | 2.0 ± 1.2 | 21.8 ± 1.3 |
| b2, mV/pH | 4.6 ± 0.7 | insignificant | 2.56 ± 0.17 | 3.45 ± 0.12 | 3.6 ± 0.3 | 6.6 ± 0.4 |
| b3, mV/pAsp | −53 ± 3 | −35 ± 3 | −45.8 ± 0.7 | −54.5 ± 0.4 | −43.9 ± 1.5 | −60.2 ± 1.6 |
| δ (K+), % | 0.8–21 | 0.2–16 | 0.12–7 | |||
| δ (Asp−, Asp±), % | 0.2–19 | 0.5–14 | 0.07–20 | |||
| sr (K+), % | 7–22 | 7–21 | 3–17 | |||
| sr (Asp−, Asp±), % | 3–20 | 0.3–8 | 0.4–15 | |||
| ctheor., M | pH | −∆φD, MB | cexp, M | δ, % | sr, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Asp−, Asp± | DP-sensor 1 | DP-sensor 2 | K+ | Asp−, Asp± | K+ | Asp−, Asp± | K+ | Asp−, Asp± | |
| 1.254 × 10−4 | 2.364 × 10−4 | 5.96 ± 0.06 | 182 ± 7 | 184 ± 3 | (1.2 ± 0.2) × 10−4 | (2.48 ± 0.18) × 10−4 | 1.5 | 5 | 17 | 7 |
| 1.254 × 10−3 | 2.364 × 10−3 | 6.21 ± 0.08 | 142 ± 4 | 142 ± 2 | (1.21 ± 0.04) × 10−3 | (2.01 ± 0.15) × 10−3 | 3 | 15 | 4 | 9 |
| 1.254 × 10−2 | 2.364 × 10−2 | 6.58 ± 0.04 | 92.1 ± 0.9 | 90.4 ± 1.5 | (1.23 ± 0.11) × 10−2 | (2.40 ± 0.05) × 10−2 | 2 | 1.5 | 11 | 3 |
| cexp, M (Solution) | cexp, mg/mL (Panangin®) | cmean, mg/mL (Panangin®) | δ, % | cexp, mg/mL (Panangin®) | cmean, mg/mL (Panangin®) | δ, % | |
|---|---|---|---|---|---|---|---|
| K+ | Asp−, Asp± | Potassium Asparaginate Hemihydrate | Magnesium Asparaginate Tetrahydrate | ||||
| (1.2 ± 0.2) × 10−4 | (2.48 ± 0.18) × 10−4 | 44 ± 7 | 44 | 2 | 45 ± 14 | 38 | 4 |
| (1.21 ± 0.04) × 10−3 | (2.01 ± 0.15) × 10−3 | 43.8 ± 1.6 | 29 ± 7 | ||||
| (1.23 ± 0.11) × 10−2 | (2.40 ± 0.05) × 10−2 | 44 ± 4 | 42 ± 5 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshina, A.; Kolganova, T.; Safronova, E.; Osipov, A.; Lapshina, E.; Yelnikova, A.; Bobreshova, O.; Yaroslavtsev, A. Perfluorosulfonic Acid Membranes Thermally Treated and Modified by Dopants with Proton-Acceptor Properties for Asparaginate and Potassium Ions Determination in Pharmaceuticals. Membranes 2019, 9, 142. https://doi.org/10.3390/membranes9110142
Parshina A, Kolganova T, Safronova E, Osipov A, Lapshina E, Yelnikova A, Bobreshova O, Yaroslavtsev A. Perfluorosulfonic Acid Membranes Thermally Treated and Modified by Dopants with Proton-Acceptor Properties for Asparaginate and Potassium Ions Determination in Pharmaceuticals. Membranes. 2019; 9(11):142. https://doi.org/10.3390/membranes9110142
Chicago/Turabian StyleParshina, Anna, Tatyana Kolganova, Ekaterina Safronova, Alexander Osipov, Ekaterina Lapshina, Anastasia Yelnikova, Olga Bobreshova, and Andrey Yaroslavtsev. 2019. "Perfluorosulfonic Acid Membranes Thermally Treated and Modified by Dopants with Proton-Acceptor Properties for Asparaginate and Potassium Ions Determination in Pharmaceuticals" Membranes 9, no. 11: 142. https://doi.org/10.3390/membranes9110142
APA StyleParshina, A., Kolganova, T., Safronova, E., Osipov, A., Lapshina, E., Yelnikova, A., Bobreshova, O., & Yaroslavtsev, A. (2019). Perfluorosulfonic Acid Membranes Thermally Treated and Modified by Dopants with Proton-Acceptor Properties for Asparaginate and Potassium Ions Determination in Pharmaceuticals. Membranes, 9(11), 142. https://doi.org/10.3390/membranes9110142