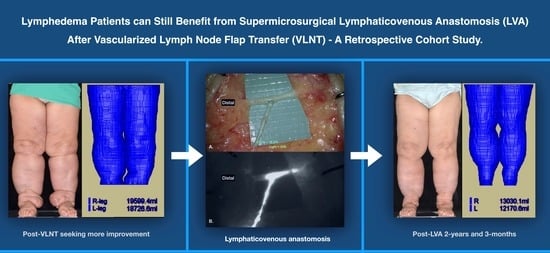
Lower Limb Lymphedema Patients Can Still Benefit from Supermicrosurgical Lymphaticovenous Anastomosis (LVA) after Vascularized Lymph Node Flap Transfer (VLNT) as Delayed Lymphatic Reconstruction—A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Operative Technique
2.2. Magnetic Resonance Volumetry (Structural Magnetic Resonance Image Acquisition and Volume Calculation) for Lower Limbs
2.3. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Intraoperative Findings
3.3. Post-LVA Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rockson, S.G.; Rivera, K.K. Estimating the Population Burden of Lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 147–154. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Coroneos, C.J. Surgical Treatment of Lymphedema. Plast. Reconstr. Surg. 2019, 144, 738–758. [Google Scholar] [CrossRef]
- Hassan, K.; Chang, D.W. The Charles Procedure as Part of the Modern Armamentarium Against Lymphedema. Ann. Plast. Surg. 2020, 85, e37–e43. [Google Scholar] [CrossRef]
- Karri, V.; Yang, M.-C.; Lee, I.J.; Chen, S.-H.; Hong, J.P.; Xu, E.-S.; Cruz-Vargas, J.; Chen, H.-C. Optimizing Outcome of Charles Procedure for Chronic Lower Extremity Lymphoedema. Ann. Plast. Surg. 2011, 66, 393–402. [Google Scholar] [CrossRef]
- Kung, T.A.; Champaneria, M.C.; Maki, J.H.; Neligan, P.C. Current Concepts in the Surgical Management of Lymphedema. Plast. Reconstr. Surg. 2017, 139, 1003e–1013e. [Google Scholar] [CrossRef]
- Yang, J.C.-S.; Wu, S.-C.; Lin, W.-C.; Chiang, M.-H.; Chiang, P.-L.; Hsieh, C.-H. Supermicrosurgical Lymphaticovenous Anastomosis as Alternative Treatment Option for Moderate-to-Severe Lower Limb Lymphedema. J. Am. Coll. Surg. 2020, 230, 216–227. [Google Scholar] [CrossRef]
- Cha, H.G.; Oh, T.M.; Cho, M.-J.; Pak, C.S.J.; Suh, H.P.; Jeon, J.Y.; Hong, J.P. Changing the Paradigm: Lymphovenous Anastomosis in Advanced Stage Lower Extremity Lymphedema. Plast. Reconstr. Surg. 2021, 147, 199–207. [Google Scholar] [CrossRef]
- Hara, H.; Mihara, M. Lymphaticovenous anastomosis for advanced-stage lower limb lymphedema. Microsurgery 2021, 41, 140–145. [Google Scholar] [CrossRef]
- Ciudad, P.; Agko, M.; Patel, K.M.; Torto, F.L.; Forte, A.J.; Chen, H. A single-stage triple-inset vascularized gastroepiploic lymph node transfers for the surgical treatment of extremity lymphedema. Microsurgery 2021, 41, 97–99. [Google Scholar] [CrossRef]
- Mihara, M.; Zhou, H.P.; Hara, H.; Tange, S.; Haragi, M. Case Report: A New Hybrid Surgical Approach for Treating Mosaic Pattern Secondary Lymphedema in the Lower Extremities. Ann. Vasc. Surg. 2014, 28, 1798.e1–1798.e6. [Google Scholar] [CrossRef]
- Masia, J.; Pons, G.; Nardulli, M.L. Combined Surgical Treatment in Breast Cancer-Related Lymphedema. J. Reconstr. Microsurg. 2015, 32, 016–027. [Google Scholar] [CrossRef] [Green Version]
- Sapountzis, S.; Ciudad, P.; Chen, H.-C.; Lim, S.Y.; Chilgar, R.M.; Kiranantawat, K.; Nicoli, F.; Constantinides, J.; Wei, M.Y.S.; Sönmez, T.T.; et al. Modified Charles procedure and lymph node flap transfer for advanced lower extremity lymphedema. Microsurgery 2014, 34, 439–447. [Google Scholar] [CrossRef]
- Ciudad, P.; Agko, M.; Huang, T.C.T.; Manrique, O.J.; Chang, W.-L.; Nicoli, F.; Maruccia, M.; Torto, F.L.; Chen, H.-C. Comprehensive multimodal surgical treatment of end-stage lower extremity lymphedema with toe management: The combined Charles,’ Homan’s, and vascularized lymph node transfer (CHAHOVA) procedures. J. Surg. Oncol. 2019, 119, 430–438. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshimatsu, H.; Yamamoto, N. Complete lymph flow reconstruction: A free vascularized lymph node true perforator flap transfer with efferent lymphaticolymphatic anastomosis. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1227–1233. [Google Scholar] [CrossRef]
- Akita, S.; Yamaji, Y.; Tokumoto, H.; Sasahara, Y.; Kubota, Y.; Kuriyama, M.; Mitsukawa, N. Improvement of the efficacy of vascularized lymph node transfer for lower-extremity lymphedema via a prefabricated lympho-venous shunt through lymphaticovenular anastomosis between the efferent lymphatic vessel and small vein in the elevated vascularized. Microsurgery 2017, 38, 270–277. [Google Scholar] [CrossRef]
- Granzow, J.W.; Soderberg, J.M.; Dauphine, C. A Novel Two-Stage Surgical Approach to Treat Chronic Lymphedema. Breast J. 2014, 20, 420–422. [Google Scholar] [CrossRef]
- Forte, A.J.; Huayllani, M.T.; Boczar, D.; Ciudad, P.; Manrique, O. Lipoaspiration and Lymph Node Transfer for Treatment of Breast Cancer-related Lymphedema: A Systematic Review. Cureus 2019, 11, e6096. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.; Chang, D.W. Vascularized lymph node transfer and lymphovenous bypass: Novel treatment strategies for symptomatic lymphedema. J. Surg. Oncol. 2016, 113, 932–939. [Google Scholar] [CrossRef]
- Raju, A.; Chang, D.W. Vascularized Lymph Node Transfer for Treatment of Lymphedema. Ann. Surg. 2015, 261, 1013–1023. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Yoshimatsu, H.; Narushima, M.; Koshima, I. Factors Associated with Lymphosclerosis. Plast. Reconstr. Surg. 2017, 140, 734–741. [Google Scholar] [CrossRef]
- Tsai, P.-L.; Wu, S.-C.; Lin, W.-C.; Mito, D.; Chiang, M.-H.; Hsieh, C.-H.; Yang, J.C.-S. Determining factors in relation to lymphovascular characteristics and anastomotic configuration in supermicrosurgical lymphaticovenous anastomosis–A retrospective cohort study. Int. J. Surg. 2020, 81, 39–46. [Google Scholar] [CrossRef]
- Ozturk, C.N.; Ozturk, C.; Glasgow, M.; Platek, M.; Ashary, Z.; Kuhn, J.; Aronoff, N.; Lohman, R.; Djohan, R.; Gurunluoglu, R. Free vascularized lymph node transfer for treatment of lymphedema: A systematic evidence based review. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1234–1247. [Google Scholar] [CrossRef]
- Gould, D.J.; Mehrara, B.J.; Neligan, P.; Cheng, M.; Patel, K.M. Lymph node transplantation for the treatment of lymphedema. J. Surg. Oncol. 2018, 118, 736–742. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Badash, I.; Selber, J.C.; Cheng, M.-H.; Patel, K.M. Vascularized Lymph Node Transfer for Lymphedema. Semin. Plast. Surg. 2018, 32, 028–035. [Google Scholar] [CrossRef]
- Pappalardo, M.; Patel, K.; Cheng, M.-H. Vascularized lymph node transfer for treatment of extremity lymphedema: An overview of current controversies regarding donor sites, recipient sites and outcomes. J. Surg. Oncol. 2018, 117, 1420–1431. [Google Scholar] [CrossRef]
- Ciudad, P.; Date, S.; Manrique, O.J.; Chang, W.-L.; Huang, T.-C.; Chen, T.-W.; Nicoli, F.; Maruccia, M.; Chen, H.-C. Recurrent Advanced Lower Extremity Lymphedema Following Initial Successful Vascularized Lymph Node Transfer: A Clinical and Histopathological Analysis. Arch. Plast. Surg. 2017, 44, 87–89. [Google Scholar] [CrossRef] [Green Version]
- Suami, H.; Scaglioni, M.F.; Dixon, K.A.; Tailor, R.C. Interaction between vascularized lymph node transfer and recipient lymphatics after lymph node dissection—a pilot study in a canine model. J. Surg. Res. 2016, 204, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.H.; Chou, P.-Y.; Hsieh, Y.-H.; Momeni, A.; Fang, Y.-H.D.; Patel, K.M.; Yang, C.-Y.; Cheng, M.-H. Quantity of lymph nodes correlates with improvement in lymphatic drainage in treatment of hind limb lymphedema with lymph node flap transfer in rats. Microsurgery 2015, 36, 239–245. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Ho, O.A.; Cheng, M.-H.; Hsiao, H.-Y. Critical Ischemia Time, Perfusion, and Drainage Function of Vascularized Lymph Nodes. Plast. Reconstr. Surg. 2018, 142, 688–697. [Google Scholar] [CrossRef]
- Tinhofer, I.E.; Yang, C.; Chen, C.; Cheng, M. Impacts of arterial ischemia or venous occlusion on vascularized groin lymph nodes in a rat model. J. Surg. Oncol. 2019, 121, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Perrault, D.P.; Lee, G.K.; Bouz, A.; Sung, C.; Yu, R.; Pourmoussa, A.J.; Park, S.Y.; Kim, G.H.; Jiao, W.; Patel, K.M.; et al. Ischemia and reperfusion injury in superficial inferior epigastric artery-based vascularized lymph node flaps. PLoS ONE 2020, 15, e0227599. [Google Scholar] [CrossRef]
- Brorson, H. Liposuction in Lymphedema Treatment. J. Reconstr. Microsurg. 2016, 32, 056–065. [Google Scholar] [CrossRef]
- Kaoutzanis, C.; Gupta, V.; Winocour, J.; Layliev, J.; Ramirez, R.; Grotting, J.C.; Higdon, K. Cosmetic Liposuction: Preoperative Risk Factors, Major Complication Rates, and Safety of Combined Procedures. Aesthetic Surg. J. 2017, 37, 680–694. [Google Scholar] [CrossRef]
- Chen, S.-H.; Yildirim, M.E.C.; Mousavi, S.A.; Chen, H.-C. Long-term functional outcomes upon application of split-thickness skin graft around major joints in HCC (Hung-Chi Chen)-modified Charles’ procedure for advanced lymphedema. Asian J. Surg. 2021, 44, 169–173. [Google Scholar] [CrossRef]
- Vignes, S.; Blanchard, M.; Yannoutsos, A.; Arrault, M. Complications of Autologous Lymph-node Transplantation for Limb Lymphoedema. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 516–520. [Google Scholar] [CrossRef] [Green Version]
- Demiri, E.; Dionyssiou, D.; Tsimponis, A.; Goula, O.C.; Mιlothridis, P.; Pavlidis, L.; Spyropoulou, G.A.; Foroglou, P. Donor-Site Lymphedema Following Lymph Node Transfer for Breast Cancer-Related Lymphedema: A Systematic Review of the Literature. Lymphat. Res. Biol. 2018, 16, 2–8. [Google Scholar] [CrossRef]
- Pons, G.; Masia, J.; Loschi, P.; Nardulli, M.L.; Duch, J. A case of donor-site lymphoedema after lymph node–superficial circumflex iliac artery perforator flap transfer. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 119–123. [Google Scholar] [CrossRef]
- Ciudad, P.; Manrique, O.J.; Bustos, S.; Agko, M.; Huang, T.C.-T.; Vizcarra, L.; Nuñez, M.L.; Torto, F.L.; Forte, A.J. Single-stage VASER-assisted liposuction and lymphatico-venous anastomoses for the treatment of extremity lymphedema: A case series and systematic review of the literature. Gland. Surg. 2020, 9, 545–557. [Google Scholar] [CrossRef]




| Sex, female/male, n (%) | 7 (87.5)/1 (12.5) |
| Age, year, median (IQR) | 69.5 (58.8–71.3) |
| Etiology, Gynecologic Cancers */sarcoma, n (%) | 7 (87.5)/1 (12.5) |
| ISL Staging (0–I/II–III), n (%) | 1 (12.5)/7 (87.5) |
| BMI, kg/m2, median (IQR) | 26.5 (22.3–33.6) |
| DM, yes/no, n (%) | 2 (25)/6 (75) |
| HTN, yes/no, n (%) | 3 (37.5)/5 (62.5) |
| Affected limb (Left/Right, bilateral), n (%) | 3 (37.5)/4 (50)/1 (12.5) |
| Chemotherapy, yes/no, n (%) | 3 (37.5)/5 (62.5) |
| Radiotherapy, yes/no, n (%) | 4 (50)/4 (50) |
| Duration of LE, year, median (IQR) | 10.5 (4.9–15.3) |
| Cellulitis episode before vs. after LVA, n, median (IQR) | 2 (1–12) vs. 0.00 (0–1.5), p = 0.047 |
| Donor site, VLNT, n £ | Five submental Three supraclavicular One omentum |
| Recipient sites, VLNT | All located distally, near medial malleolus region |
| Time between VLNT and LVA, month, median (IQR) | 41.4 (22.3–97.9) |
| Volume gained in the LE Limb @, mL, median (IQR) | 3836 (2505–4584) |
| Total LVs found | 72 |
| Incisions per patient, median (IQR) | 4 (3–5) |
| LVs found per patient, median (IQR) | 8 (7–9) |
| Diameter of LVs, mm, median (IQR) | 0.6 (0.4–0.7) |
| LVA performed per patient, median (IQR) | 8 (7–9) |
| Total number (percentage) of ICG (+) LVs, n (%) | 57 (79.2) |
| Diameter, mm, median (IQR) | 0.6 (0.4–0.8) |
| Total number (percentage) of Flow (+) LVs, n (%) | 64 (88.9) |
| Diameter, mm, median (IQR) | 0.6 (0.5–0.8) |
| Lymphosclerosis Classification, n, (%) | |
| s0 s1 s2 s3 | 8 (11.1) 36 (50.0) 26 (36.1) 2 (2.8) |
| Total number of recipient veins | 42 |
| Recipient Veins per Patient, median (IQR) | 5 (4–6) |
| Diameter, mm, median (IQR) | 0.8 (0.8–1.0) |
| Operative Time (min), LVA, median (IQR) | 455.5 (389.0–510.0) |
| Kruskal−Wallis Rank Sum Test | Mann−Whitney Wilcoxon Test | ||
|---|---|---|---|
| Post-LVA follow-up, month, median (IQR) | 18 (6–30) | - | - |
| Six-Months Post-LVA Volume Reduction *, mL, median (IQR) | 522 (429–1644) | H0: (pre-LVA) = (6-Months Post-LVA Volume Reduction **, %) = (1-Year Post-LVA Volume Reduction **, %) p < 0.001 | - |
| Six-Months Post-LVA Volume Reduction **, %, median (IQR) | 20.9 (15.3–29.8) | p < 0.001 | |
| One-Year Post-LVA Volume Reduction *, mL, median (IQR) | 1943 (603–3674) | - | |
| One-Year Post-LVA Volume Reduction **, %, median (IQR) | 31.0 (16.5–32.1) | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.C.-S.; Wu, S.-C.; Hayashi, A.; Lin, W.-C.; Huang, G.-K.; Tsai, P.-Y.; Chien, P.-C.; Hsieh, C.-H. Lower Limb Lymphedema Patients Can Still Benefit from Supermicrosurgical Lymphaticovenous Anastomosis (LVA) after Vascularized Lymph Node Flap Transfer (VLNT) as Delayed Lymphatic Reconstruction—A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 3121. https://doi.org/10.3390/jcm10143121
Yang JC-S, Wu S-C, Hayashi A, Lin W-C, Huang G-K, Tsai P-Y, Chien P-C, Hsieh C-H. Lower Limb Lymphedema Patients Can Still Benefit from Supermicrosurgical Lymphaticovenous Anastomosis (LVA) after Vascularized Lymph Node Flap Transfer (VLNT) as Delayed Lymphatic Reconstruction—A Retrospective Cohort Study. Journal of Clinical Medicine. 2021; 10(14):3121. https://doi.org/10.3390/jcm10143121
Chicago/Turabian StyleYang, Johnson Chia-Shen, Shao-Chun Wu, Akitatsu Hayashi, Wei-Che Lin, Gong-Kai Huang, Pei-Yu Tsai, Peng-Chen Chien, and Ching-Hua Hsieh. 2021. "Lower Limb Lymphedema Patients Can Still Benefit from Supermicrosurgical Lymphaticovenous Anastomosis (LVA) after Vascularized Lymph Node Flap Transfer (VLNT) as Delayed Lymphatic Reconstruction—A Retrospective Cohort Study" Journal of Clinical Medicine 10, no. 14: 3121. https://doi.org/10.3390/jcm10143121
APA StyleYang, J. C. -S., Wu, S. -C., Hayashi, A., Lin, W. -C., Huang, G. -K., Tsai, P. -Y., Chien, P. -C., & Hsieh, C. -H. (2021). Lower Limb Lymphedema Patients Can Still Benefit from Supermicrosurgical Lymphaticovenous Anastomosis (LVA) after Vascularized Lymph Node Flap Transfer (VLNT) as Delayed Lymphatic Reconstruction—A Retrospective Cohort Study. Journal of Clinical Medicine, 10(14), 3121. https://doi.org/10.3390/jcm10143121