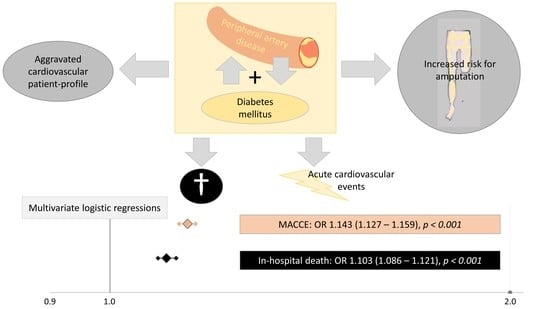
Diabetes Mellitus and Its Impact on Patient-Profile and In-Hospital Outcomes in Peripheral Artery Disease
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Endpoints and Adverse in-Hospital Events
Definitions
2.2. Ethical Aspects
2.3. Statistical Methods
- Adjustment I: age, sex, cancer, heart failure, coronary artery disease (CAD), chronic obstructive pulmonary disease, essential arterial hypertension, acute and chronic kidney disease, atrial fibrillation/flutter (AF), and hyperlipidemia.
- Adjustment II: age, sex, cancer, heart failure, CAD, chronic obstructive pulmonary disease, essential arterial hypertension, acute and chronic kidney disease, AF, hyperlipidemia and treatment year.
3. Results
3.1. Clinical Profile of PAD Patients with and without DM
3.2. Influence of DM on Outcomes in PAD Patients
3.3. Influence of DM on Amputation Surgeries in Patients with PAD
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| AF | Atrial fibrillation/flutter |
| CAD | Coronary artery disease |
| CI | Confidence interval |
| CPR | Cardiopulmonary resuscitation |
| DM | Diabetes mellitus |
| DRG | Diagnosis Related Groups |
| DVT | Deep venous thrombosis or thrombophlebitis of the leg veins |
| GIB | Gastro-intestinal bleeding |
| ICB | Intracerebral bleeding events |
| ICD | International Classification of Diseases and Related Health Problems |
| IQR | Inter-quartile range |
| OPS | Diagnostic, surgery and procedures codes (Operationen und Prozedurenschlüssel) |
| OR | Odds ratio |
| MACCE | Major adverse cardiovascular and cerebrovascular events |
| PAD | Peripheral artery disease |
| PE | Pulmonary embolism |
| RDC | Research Data Center |
| vs. | Versus |
References
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef] [Green Version]
- Criqui, M.H.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [Green Version]
- Soyoye, D.O.; Abiodun, O.O.; Ikem, R.T.; Kolawole, B.A.; Akintomide, A.O. Diabetes and peripheral artery disease: A review. World J. Diabetes 2021, 12, 827–838. [Google Scholar] [CrossRef]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Silbernagel, G.; Rein, P.; Saely, C.H.; Engelberger, R.P.; Willenberg, T.; Do, D.-D.; Kucher, N.; Baumgartner, I.; Drexel, H. Prevalence of type 2 diabetes is higher in peripheral artery disease than in coronary artery disease patients. Diabetes Vasc. Dis. Res. 2015, 12, 146–149. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, V.H.; Hobohm, L.; Münzel, T.; Wenzel, P.; Gori, T.; Keller, K. Impact of diabetes mellitus on mortality rates and outcomes in myocardial infarction. Diabetes Metab. 2021, 47, 101211. [Google Scholar] [CrossRef]
- Cea-Soriano, L.; Fowkes, F.G.R.; Johansson, S.; Allum, A.M.; Rodriguez, L.A.G. Time trends in peripheral artery disease incidence, prevalence and secondary preventive therapy: A cohort study in The Health Improvement Network in the UK. BMJ Open 2018, 8, e018184. [Google Scholar] [CrossRef] [Green Version]
- Collaboration, NCD Risk Factor. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Hiatt, W.R. Peripheral Arterial Disease in Patients With Diabetes. J. Am. Coll. Cardiol. 2006, 47, 921–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahara, M. Diabetes Mellitus and Lower Extremity Peripheral Artery Disease. JMA J. 2021, 4, 225–231. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.L. Update on Some Epidemiologic Features of Intermittent Claudication: The Framingham Study. J. Am. Geriatr. Soc. 1985, 33, 13–18. [Google Scholar] [CrossRef]
- Kamil, S.; Sehested, T.S.G.; Carlson, N.; Houlind, K.; Lassen, J.F.; Bang, C.N.; Dominguez, H.; Pedersen, C.T.; Gislason, G.H. Diabetes and risk of peripheral artery disease in patients undergoing first-time coronary angiography between 2000 and 2012—A nationwide study. BMC Cardiovasc. Disord. 2019, 19, 234. [Google Scholar] [CrossRef] [Green Version]
- Golledge, J.; Drovandi, A.; Rowbotham, S.; Velu, R.; Quigley, F.; Jenkins, J. Control of modifiable risk factors and major adverse cardiovascular events in people with peripheral artery disease and diabetes. World J. Diabetes 2021, 12, 883–892. [Google Scholar] [CrossRef]
- Olesen, K.K.W.; Gyldenkerne, C.; Thim, T.; Thomsen, R.W.; Maeng, M. Peripheral artery disease, lower limb revascularization, and amputation in diabetes patients with and without coronary artery disease: A cohort study from the Western Denmark Heart Registry. BMJ Open Diabetes Res. Care 2021, 9, e001803. [Google Scholar] [CrossRef]
- Luan, J.; Xu, J.; Zhong, W.; Zhou, Y.; Liu, H.; Qian, K. Adverse Prognosis of Peripheral Artery Disease Treatments Associated With Diabetes: A Comprehensive Meta-Analysis. Angiology 2021, 2021. [Google Scholar] [CrossRef]
- Jacob-Brassard, J.; Al-Omran, M.; Hussain, M.A.; Mamdani, M.; Stukel, T.A.; Lee, D.S.; de Mestral, C. Temporal Trends in Hospitalization for Lower Extremity Peripheral Artery Disease in Ontario: The Importance of Diabetes. Can. J. Cardiol. 2021. [Google Scholar] [CrossRef]
- Kamitani, F.; Nishioka, Y.; Noda, T.; Myojin, T.; Kubo, S.; Higashino, T.; Okada, S.; Akai, Y.; Ishii, H.; Takahashi, Y.; et al. Incidence of lower limb amputation in people with and without diabetes: A nationwide 5-year cohort study in Japan. BMJ Open 2021, 11, e048436. [Google Scholar] [CrossRef]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef]
- Kaneko, M.; Fujihara, K.; Harada, M.Y.; Osawa, T.; Yamamoto, M.; Kitazawa, M.; Matsubayashi, Y.; Yamada, T.; Seida, H.; Kodama, S.; et al. Rates and risk factors for amputation in people with diabetes in Japan: A historical cohort study using a nationwide claims database. J. Foot Ankle Res. 2021, 14, 29. [Google Scholar] [CrossRef]
- Chatha, K.K.; Walsh, B.; La Fontaine, J.; Bowen, M.E.; Meneghini, L. Lower-Extremity Amputation Trends Among People With Diabetes in a Large Urban Environment. Diabetes Care 2021, 44, e91–e92. [Google Scholar] [CrossRef]
- Cascini, S.; Agabiti, N.; Davoli, M.; Uccioli, L.; Meloni, M.; Giurato, L.; Marino, C.; Bargagli, A.M. Survival and factors predicting mortality after major and minor lower-extremity amputations among patients with diabetes: A population-based study using health information systems. BMJ Open Diabetes Res. Care 2020, 8, e001355. [Google Scholar] [CrossRef]
- Reinecke, H.; Unrath, M.; Freisinger, E.; Bunzemeier, H.; Meyborg, M.; Lüders, F.; Gebauer, K.; Roeder, N.; Berger, K.; Malyar, N.M. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015, 36, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malyar, N.; Fürstenberg, T.; Wellmann, J.; Meyborg, M.; Lüders, F.; Gebauer, K.; Bunzemeier, H.; Roeder, N.; Reinecke, H. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: A nationwide population-based analysis. Eur. Heart J. 2013, 34, 2706–2714. [Google Scholar] [CrossRef] [Green Version]
- Kreutzburg, T.; Peters, F.; Riess, H.C.; Hischke, S.; Marschall, U.; Kriston, L.; L’Hoest, H.; Sedrakyan, A.; Debus, E.S.; Behrendt, C.A. Editor’s Choice—Comorbidity Patterns Among Patients with Peripheral Arterial Occlusive Disease in Germany: A Trend Analysis of Health Insurance Claims Data. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Deutsche Kodierrichtlinien 2018 Druckversion A4 (PDF). 2018. Available online: https://www.g-drg.de/inek_site_de/layout/set/standard/Media/Files/G-DRG-System/G-DRG-System_2018/Deutsche_Kodierrichtlinien_2018_Druckversion_A4_PDF (accessed on 5 June 2020).
- Keller, K.; Hobohm, L.; Münzel, T.; Ostad, M.A. Sex-specific differences regarding seasonal variations of incidence and mortality in patients with myocardial infarction in Germany. Int. J. Cardiol. 2019, 287, 132–138. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Ebner, M.; Kresoja, K.-P.; Münzel, T.; Konstantinides, S.V.; Lankeit, M. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur. Heart J. 2020, 41, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galie, N.; Gibbs, J.S.R.; Huisman, M.V.; Humbert, M.; Kucher, N. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3069, 69a–69k. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2019, 41, 543–603. [Google Scholar] [CrossRef]
- Perkins, G.D.; Handley, A.J.; Koster, R.W.; Castrén, M.; Smyth, M.A.; Olasveengen, T.; Monsieurs, K.G.; Raffay, V.; Gräsner, J.T.; Wenzel, V.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation 2015, 95, 81–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröger, K.; Berg, C.; Santosa, F.; Malyar, N.; Reinecke, H. Lower Limb Amputation in Germany. Dtsch. Arztebl. Int. 2017, 114, 130–136. [Google Scholar]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, e71–e126. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Stoberock, K.; Kaschwich, M.; Nicolay, S.S.; Mahmoud, N.; Heidemann, F.; Rieß, H.C.; Debus, E.S.; Behrendt, C.-A. The interrelationship between diabetes mellitus and peripheral arterial disease. Vasa 2021, 50, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.A.; Varga-Szemes, A.; Schoepf, U.J.; Emrich, T.; Schwarz, F.; Kroencke, T.J.; Scheurig-Muenkler, C. In-patient care trends in peripheral artery disease in the German healthcare system over the past decade. Eur. Radiol. 2021, 1–12. [Google Scholar] [CrossRef]
- Karbach, S.; Hobohm, L.; Wild, J.; Münzel, T.; Gori, T.; Wegner, J.; Steinbrink, K.; Wenzel, P.; Keller, K. Impact of Psoriasis on Mortality Rate and Outcome in Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e016956. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Hobohm, L.; Ostad, M.A.; Göbel, S.; Lankeit, M.; Konstantinides, S.; Münzel, T.; Wenzel, P. Temporal trends and predictors of inhospital death in patients hospitalised for heart failure in Germany. Eur. J. Prev. Cardiol. 2020, 28, 990–997. [Google Scholar] [CrossRef]
- Richter, L.; Freisinger, E.; Lüders, F.; Gebauer, K.; Meyborg, M.; Malyar, N.M. Impact of diabetes type on treatment and outcome of patients with peripheral artery disease. Diabetes Vasc. Dis. Res. 2018, 15, 504–510. [Google Scholar] [CrossRef]
- Thiruvoipati, T.; Kielhorn, C.E.; Armstrong, E.J. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J. Diabetes 2015, 6, 961–969. [Google Scholar] [CrossRef]
- Hooi, J.D.; Kester, A.D.; Stoffers, H.E.; Overdijk, M.M.; Van Ree, J.W.; Knottnerus, J.A. Incidence of and Risk Factors for Asymptomatic Peripheral Arterial Occlusive Disease: A Longitudinal Study. Am. J. Epidemiol. 2001, 153, 666–672. [Google Scholar] [CrossRef]
- Dagenais, G.R.; Maurice, S.; Robitaille, N.M.; Gingras, S.; Lupien, P.J. Intermittent claudication in Quebec men from 1974-1986: The Quebec Cardiovascular Study. Clin. Investig. Med. 1991, 14, 93–100. [Google Scholar]
- Fowkes, F.G.; Housley, E.; Riemersma, R.A.; MacIntyre, C.C.; Cawood, E.H.; Prescott, R.J.; Ruckley, C.V. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am. J. Epidemiol. 1992, 135, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.; Vaccaro, O.; Neaton, J.D.; Wentworth, D. Diabetes, Other Risk Factors, and 12-Yr Cardiovascular Mortality for Men Screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993, 16, 434–444. [Google Scholar] [CrossRef]
- Epstein, M.; Sowers, J.R. Diabetes mellitus and hypertension. Hypertension 1992, 19, 403–418. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.S.; Patel, M.R.; Dai, D.; Subherwal, S.; Stafford, J.; Calhoun, S.; Peterson, E.D. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: Results from U.S. Medicare 2000–2008. J. Am. Coll. Cardiol. 2012, 60, 2230–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrsalovic, M.; Vucur, K.; Vrsalovic Presecki, A.; Fabijanic, D.; Milosevic, M. Impact of diabetes on mortality in peripheral artery disease: A meta-analysis. Clin. Cardiol. 2017, 40, 287–291. [Google Scholar] [CrossRef]
- Noskin, G.A.; Rubin, R.J.; Schentag, J.J.; Kluytmans, J.; Hedblom, E.C.; Smulders, M.; Lapetina, E.; Gemmen, E. The burden of Staphylococcus aureus infections on hospitals in the United States: An analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch. Intern. Med. 2005, 165, 1756–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameters | PAD Patients with Diabetes (n = 864,691; 32.6%) | PAD Patients without Diabetes (n = 1,790,180; 67.4%) | p-Value † |
|---|---|---|---|
| Age | 73.0 (65.0–79.0) | 71.0 (62.0–79.0) | <0.001 |
| Age ≥ 70 years | 512,438 (59.3%) | 928,893 (51.9%) | <0.001 |
| Female sex * | 296,102 (34.2%) | 679,226 (37.9%) | <0.001 |
| In-hospital stay (days) | 8.0 (3.0–16.0) | 6.0 (2.0–13.0) | <0.001 |
| Diabetes mellitus subtypes | |||
| Diabetes mellitus type I | 19,799 (2.3%) | ||
| Diabetes mellitus type II | 833,780 (96.4%) | ||
| Unknown/uncoded diabetes subtype | 11,112 (1.3%) | ||
| Traditional cardiovascular risk factors | |||
| Obesity | 103,253 (11.9%) | 98,913 (5.5%) | <0.001 |
| Essential arterial hypertension | 596,842 (69.0%) | 1,083,759 (60.5%) | <0.001 |
| Hyperlipidaemia | 331,467 (38.3%) | 594,525 (33.2%) | <0.001 |
| Comorbidities | |||
| Cancer | 14,523 (1.7%) | 32,814 (1.8%) | <0.001 |
| Coronary artery disease | 308,501 (35.7%) | 421,655 (23.6%) | <0.001 |
| Heart failure | 143,658 (16.6%) | 187,766 (10.5%) | <0.001 |
| Atrial fibrillation/flutter | 161,814 (18.7%) | 235,373 (13.1%) | <0.001 |
| Chronic obstructive pulmonary disease | 76,858 (8.9%) | 166,201 (9.3%) | <0.001 |
| Acute and chronic kidney disease | 308,985 (35.7%) | 363,763 (20.3%) | <0.001 |
| Anaemia | 148,485 (17.2%) | 234,460 (13.1%) | <0.001 |
| Charlson comorbidity index | 6.0 (5.0–8.0) | 4.0 (3.0–5.0) | <0.001 |
| Amputation treatment | |||
| Amputation | 141,742 (16.4%) | 163,099 (9.1%) | <0.001 |
| Minor amputation | 101,681 (11.8%) | 101,271 (5.7%) | <0.001 |
| Major amputation | 52,365 (6.1%) | 71,067 (4.0%) | <0.001 |
| Adverse events during hospitalization | |||
| In-hospital death | 30,129 (3.5%) | 46,587 (2.6%) | <0.001 |
| MACCE | 40,760 (4.7%) | 59,327 (3.3%) | <0.001 |
| Cardio-pulmonary resuscitation | 7416 (0.9%) | 9221 (0.5%) | <0.001 |
| Shock | 7610 (0.9%) | 11,884 (0.7%) | <0.001 |
| Myocardial infarction | 10,685 (1.2%) | 12,082 (0.7%) | <0.001 |
| Pulmonary embolism | 1,151 (0.13%) | 2,053 (0.11%) | <0.001 |
| Deep venous thrombosis or thrombophlebitis | 4767 (0.55%) | 10,561 (0.59%) | <0.001 |
| Pneumonia | 19,305 (2.2%) | 28,103 (1.6%) | <0.001 |
| Acute kidney injury | 21,617 (2.5%) | 27,023 (1.5%) | <0.001 |
| Stroke (ischaemic or haemorrhagic) | 5499 (0.6%) | 7621 (0.4%) | <0.001 |
| Intracerebral bleeding | 545 (0.03%) | 277 (0.03%) | 0.504 |
| Gastro-intestinal bleeding | 4066 (0.5%) | 7091 (0.4%) | <0.001 |
| Transfusion of blood constituents | 107,710 (12.5%) | 178,379 (10.0%) | <0.001 |
| Univariable Regression Model | Multivariable Regression Model (Adjustment I) | Multivariable Regression Model (Adjustment II) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| In-hospital death | 1.351 (1.331–1.371) | <0.001 | 1.077 (1.060–1.093) | <0.001 | 1.103 (1.086–1.121) | <0.001 |
| Cardio-pulmonary resuscitation | 1.671 (1.620–1.723) | <0.001 | 1.189 (1.152–1.228) | <0.001 | 1.203 (1.165–1.242) | <0.001 |
| MACCE | 1.443 (1.425–1.462) | <0.001 | 1.118 (1.103–1.133) | <0.001 | 1.143 (1.127–1.159) | <0.001 |
| Pulmonary embolism | 1.161 (1.080–1.248) | <0.001 | 1.011 (0.939–1.089) | 0.772 | 0.995 (0.924–1.073) | 0.905 |
| Pneumonia | 1.432 (1.406–1.459) | <0.001 | 1.106 (1.085–1.127) | <0.001 | 1.123 (1.101–1.145) | <0.001 |
| Deep venous thrombosis or thrombophlebitis | 0.934 (0.903–0.967) | <0.001 | 0.896 (0.865–0.929) | <0.001 | 0.887 (0.856–0.919) | <0.001 |
| Acute kidney injury | 1.673 (1.643–1.703) | <0.001 | – | – | – | – |
| Myocardial infarction | 1.841 (1.794–1.890) | <0.001 | 1.219 (1.186–1.252) | <0.001 | 1.241 (1.208–1.276) | <0.001 |
| Shock | 1.329 (1.291–1.368) | <0.001 | 0.950 (0.922–0.979) | 0.001 | 0.954 (0.926–0.983) | 0.002 |
| Stroke (ischemic or hemorrhagic) | 1.497 (1.446–1.550) | <0.001 | 1.322 (1.275–1.370) | <0.001 | 1.338 (1.291–1.387) | <0.001 |
| Intracerebral bleeding | 1.052 (0.911–1.216) | 0.490 | 0.913 (0.787–1.058) | 0.227 | 0.920 (0.792–1.067) | 0.270 |
| Gastro-intestinal bleeding | 1.188 (1.143–1.235) | <0.001 | 0.957 (0.920–0.996) | 0.032 | 0.971 (0.933–1.010) | 0.143 |
| Transfusion of blood constituents | 1.286 (1.275–1.296) | <0.001 | 1.089 (1.080–1.098) | <0.001 | 1.110 (1.100–1.119) | <0.001 |
| Amputation treatment | ||||||
| Amputation | 1.956 (1.941–1.971) | <0.001 | 1.804 (1.790–1.818) | <0.001 | 1.822 (1.808–1.837) | <0.001 |
| Minor amputation | 2.222 (2.202–2.243) | <0.001 | 2.003 (1.984–2.022) | <0.001 | 2.008 (1.989–2.027) | <0.001 |
| Major amputation | 1.559 (1.541–1.578) | <0.001 | 1.464 (1.447–1.482) | <0.001 | 1.494 (1.476–1.512) | <0.001 |
| Univariable Regression Model | Multivariable Regression Model (Adjustment II) | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| 30–39 years | 5.602 (2.422–12.957) | <0.001 | 1.458 (0.533–3.989) | 0.462 |
| 40–49 years | 2.970 (2.363–3.732) | <0.001 | 1.635 (1.265–2.112) | <0.001 |
| 50–59 years | 1.878 (1.735–2.033) | <0.001 | 1.130 (1.036–1.233) | 0.006 |
| 60–69 years | 1.535 (1.475–1.599) | <0.001 | 1.070 (1.024–1.118) | 0.002 |
| 70–79 years | 1.409 (1.373–1.446) | <0.001 | 1.092 (1.063–1.123) | <0.001 |
| 80–89 years | 1.173 (1.146–1.201) | <0.001 | 1.086 (1.059–1.113) | <0.001 |
| ≥90 years | 1.020 (0.971–1.071) | 0.435 | 1.023 (0.973–1.076) | 0.374 |
| Univariable Regression Model | Multivariable Regression Model (Adjustment II) | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| 20–29 years | 18.043 (2.485–130.988) | 0.004 | 15.955 (1.074–236.997) | 0.044 |
| 30–39 years | 3.166 (1.598–6.271) | 0.001 | 0.997 (0.431–2.306) | 0.994 |
| 40–49 years | 2.463 (2.078–2.920) | <0.001 | 1.387 (1.147–1.676) | 0.001 |
| 50–59 years | 1.954 (1.838–2.077) | <0.001 | 1.206 (1.128–1.290) | <0.001 |
| 60–69 years | 1.638 (1.585–1.692) | <0.001 | 1.149 (1.109–1.190) | <0.001 |
| 70–79 years | 1.491 (1.458–1.524) | <0.001 | 1.144 (1.118–1.172) | <0.001 |
| 80–89 years | 1.228 (1.202–1.255) | <0.001 | 1.114 (1.089–1.140) | <0.001 |
| ≥90 years | 1.035 (0.987–1.085) | 0.152 | 1.026 (0.977–1.077) | 0.305 |
| Univariable Regression Model | Multivariable Regression Model (Adjustment II) | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| 20–29 years | 2.736 (1.310–5.717) | 0.007 | 2.483 (1.097–5.623) | 0.029 |
| 30–39 years | 1.643 (1.340–2.014) | <0.001 | 1.308 (1.040–1.644) | 0.022 |
| 40–49 years | 2.800 (2.629–2.982) | <0.001 | 2.558 (2.388–2.739) | <0.001 |
| 50–59 years | 2.799 (2.726–2.875) | <0.001 | 2.595 (2.522–2.670) | <0.001 |
| 60–69 years | 2.317 (2.278–2.356) | <0.001 | 2.122 (2.084–2.160) | <0.001 |
| 70–79 years | 1.992 (1.966–2.018) | <0.001 | 1.839 (1.814–1.864) | <0.001 |
| 80–89 years | 1.524 (1.502–1.547) | <0.001 | 1.535 (1.512–1.558) | <0.001 |
| ≥90 years | 1.254 (1.211–1.298) | <0.001 | 1.301 (1.256–1.347) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, K.; Schmitt, V.H.; Vosseler, M.; Brochhausen, C.; Münzel, T.; Hobohm, L.; Espinola-Klein, C. Diabetes Mellitus and Its Impact on Patient-Profile and In-Hospital Outcomes in Peripheral Artery Disease. J. Clin. Med. 2021, 10, 5033. https://doi.org/10.3390/jcm10215033
Keller K, Schmitt VH, Vosseler M, Brochhausen C, Münzel T, Hobohm L, Espinola-Klein C. Diabetes Mellitus and Its Impact on Patient-Profile and In-Hospital Outcomes in Peripheral Artery Disease. Journal of Clinical Medicine. 2021; 10(21):5033. https://doi.org/10.3390/jcm10215033
Chicago/Turabian StyleKeller, Karsten, Volker H. Schmitt, Markus Vosseler, Christoph Brochhausen, Thomas Münzel, Lukas Hobohm, and Christine Espinola-Klein. 2021. "Diabetes Mellitus and Its Impact on Patient-Profile and In-Hospital Outcomes in Peripheral Artery Disease" Journal of Clinical Medicine 10, no. 21: 5033. https://doi.org/10.3390/jcm10215033
APA StyleKeller, K., Schmitt, V. H., Vosseler, M., Brochhausen, C., Münzel, T., Hobohm, L., & Espinola-Klein, C. (2021). Diabetes Mellitus and Its Impact on Patient-Profile and In-Hospital Outcomes in Peripheral Artery Disease. Journal of Clinical Medicine, 10(21), 5033. https://doi.org/10.3390/jcm10215033