Elderly Population with COVID-19 and the Accuracy of Clinical Scales and D-Dimer for Pulmonary Embolism: The OCTA-COVID Study
Abstract
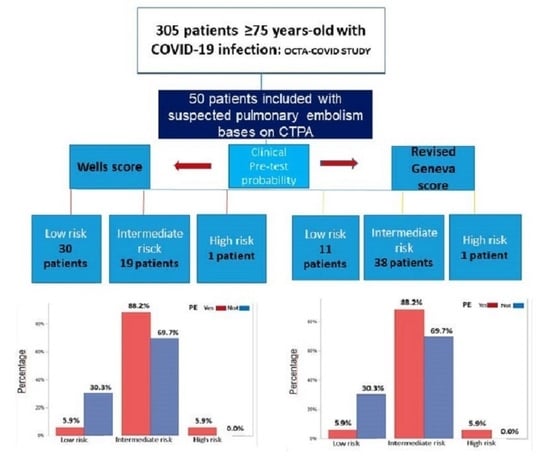
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics Approvals
2.3. Assessment of Clinical Probability
2.4. Data Collection
2.5. Laboratory Procedures
2.6. Definitions
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Predictive Values of the Wells Score and the Revised Geneva Score in the Elderly Group with COVID-19 and Pulmonary Embolism
3.3. DD and Clinical Score for the Geriatric Population with PE and COVID-19
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano-Montoya, I.; Quezada-Feijoo, M.; Jaramillo-Hidalgo, J.; Gómez-Pavón, F.J. Atypical symptoms of COVID-19 in hospitalised oldest old adults. Rev. Esp. Geriatr. Gerontol. 2021, 56, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.C.G.; Guerra, L.C.; Moro, M.G.; Alba, R.C.; García, M.P.; Camacho, M.Á.C. Pulmonary embolism in very elderly patients. A diagnostic challenge. Rev. Clín. Esp. 2019, 219, 310–314. [Google Scholar]
- Kichloo, A.; Dettloff, K.; Aljadah, M.; Albosta, M.; Jamal, S.; Singh, J.; Wani, F.; Kumar, A.; Vallabhaneni, S.; Khan, M.Z. COVID-19 and hypercoagulability: A review. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620962853. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemost. 2020, 18, 1559–1561. [Google Scholar] [CrossRef]
- Gupta, R.; Ammari, Z.; Dasa, O.; Ruzieh, M.; Burlen, J.J.; Shunnar, K.M.; Nguyen, H.T.; Xie, Y.; Brewster, P.; Chen, T.; et al. Long-term mortality after massive, submassive, and low-risk pulmonary embolism. Vasc. Med. 2020, 25, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.H.; Chen, H.L.; Chen, J.R.; Xing, J.L.; Gu, P.; Zhu, B.F. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2016, 41, 482–492. [Google Scholar] [CrossRef]
- Di Marca, S.; Cilia, C.; Campagna, A.; D’Arrigo, G.; Abd ElHafeez, S.; Tripepi, G.; Puccia, G.; Pisano, M.; Mastrosimone, G.; Terranova, V.; et al. Comparison of wells and revised geneva rule to assess pretest probability of pulmonary embolism in high-risk hospitalized elderly adults. J. Am. Geriatr. Soc. 2015, 63, 1091–1097. [Google Scholar] [CrossRef]
- Griffin, D.O.; Jensen, A.; Khan, M.; Chin, J.; Chin, K.; Saad, J.; Parnell, R.; Awwad, C.; Patel, D. Pulmonary embolism and increased levels of d-dimer in patients with coronavirus disease. Emerg. Infect. Dis. 2020, 26, 1941–1943. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Cannata, F.; Bombace, S.; Stefanini, G.G. Cardiac biomarkers in patients with COVID-19: Pragmatic tools in hard times. Rev. Esp. Cardiol. 2021, 74, 566–568. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, E.; Combescure, C.; Le Gal, G.; Nendaz, M.; Perneger, T.; Bounameaux, H.; Perrier, A.; Righini, M. Clinical prediction rules for pulmonary embolism: A systematic review and meta-analysis. J. Thromb. Haemost. 2010, 8, 957–970. [Google Scholar] [CrossRef]
- Wells, P.S.; Anderson, D.R.; Rodger, M.; Ginsberg, J.S.; Kearon, C.; Gent, M.; Turpie, A.G.; Bormanis, J.; Weitz, J.; Chamberlain, M.; et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: Increasing the models utility with the SimpliRED D-dimer. Thromb. Haemost. 2000, 83, 416–420. [Google Scholar]
- Klok, F.A.; Mos, I.C.; Nijkeuter, M.; Righini, M.; Perrier, A.; Le Gal, G.; Huisman, M.V. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch. Intern. Med. 2008, 168, 2131–2136. [Google Scholar] [CrossRef]
- McNally, M.; Curtain, J.; O’Brien, K.K.; Dimitrov, B.D.; Fahey, T. Validity of British thoracic society guidance (the CRB-65 rule) for predicting the severity of pneumonia in general practice: Systematic review and meta-analysis. Br. J. Gen. Pract. 2010, 60, e423–e433. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md State Med. J. 1965, 14, 61–65. [Google Scholar]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The global deterioration scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [Green Version]
- Apple, F.S.; Ler, R.; Murakami, M.M. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin. Chem. 2012, 58, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Quezada-Feijoo, M.; Ramos, M.; Lozano-Montoya, I.; Toro, R.; Jaramillo-Hídalgo, J.; Fernández de la Puente, E.; Garmendia, B.; Carrillo, P.; Cristofori, G.; Goñi Rosón, S.; et al. Predictive factors of pulmonary embolism in older patients with SARS-CoV-2: The OCTA-COVID-19 study. J. Clin. Med. 2021, 10, 2998. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Y-Martin, R.M.; Oldham, S.A. CTPA as the gold standard for the diagnosis of pulmonary embolism. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 557–563. [Google Scholar] [CrossRef]
- Kampouri, E.; Filippidis, P.; Viala, B.; Méan, M.; Pantet, O.; Desgranges, F.; Tschopp, J.; Regina, J.; Karachalias, E.; Bianchi, C.; et al. Predicting venous thromboembolic events in patients with coronavirus disease 2019 requiring hospitalization: An observational retrospective study by the COVIDIC initiative in a Swiss University Hospital. BioMed Res. Int. 2020, 2020, 9126148. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.T.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Wells, P.S.; Anderson, D.R.; Rodger, M.; Stiell, I.; Dreyer, J.F.; Barnes, D.; Forgie, M.; Kovacs, G.; Ward, J.; Kovacs, M.J.; et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: Management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann. Intern. Med. 2001, 135, 98–107. [Google Scholar] [CrossRef]
- Righini, M.; Perrier, A.; De Moerloose, P.; Bounameaux, H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J. Thromb. Haemost. 2008, 6, 1059–1071. [Google Scholar] [CrossRef]
- Wang, L.; Baser, O.; Wells, P.; Peacock, W.F.; Coleman, C.I.; Fermann, G.J.; Schein, J.; Crivera, C. Benefit of early discharge among patients with low-risk pulmonary embolism: Focus on net clinical impact. Circulation 2016, 134, A13112. [Google Scholar]
- Couturaud, F.; Kearon, C.; Bates, S.M.; Ginsberg, J.S. Decrease in sensitivity of D-dimer for acute venous thromboembolism after starting anticoagulant therapy. Blood Coagul. Fibrinolysis 2002, 13, 241–246. [Google Scholar] [CrossRef]
- Douma, R.A.; Le Gal, G.; Söhne, M.; Righini, M.; Kamphuisen, P.W.; Perrier, A.; Kruip, M.J.; Bounameaux, H.; Büller, H.R.; Roy, P.M. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: A retrospective analysis of three large cohorts. BMJ 2010, 340, c1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farm, M.; Siddiqui, A.; Onelöv, L.; Chaireti, R.; Holmström, M.; Antovic, J.P. Age-adjusted D-dimer cutoffs for DVT and pulmonary embolism: A comparison of five assays. Blood 2016, 128, 1419. [Google Scholar] [CrossRef]
- Alhassan, S.; Bihler, E.; Patel, K.; Lavudi, S.; Young, M.; Balaan, M. Assessment of the current D-dimer cutoff point in pulmonary embolism workup at a single institution: Retrospective study. J. Postgrad. Med. 2018, 64, 150–154. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Mestre-Gómez, B.; Lorente-Ramos, R.M.; Rogado, J.; Franco-Moreno, A.; Obispo, B.; Salazar-Chiriboga, D.; Saez-Vaquero, T.; Torres-Macho, J.; Abad-Motos, A.; Cortina-Camarero, C.; et al. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J. Thromb. Thrombolysis 2021, 51, 40–46. [Google Scholar] [CrossRef]
- Miniati, M.; Bottai, M.; Monti, S. Comparison of 3 clinical models for predicting the probability of pulmonary embolism. Medicine 2005, 84, 107–114. [Google Scholar] [CrossRef]
- Chagnon, I.; Bounameaux, H.; Aujesky, D.; Roy, P.M.; Gourdier, A.L.; Cornuz, J.; Perneger, T.; Perrier, A. Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism. Am. J. Med. 2002, 113, 269–275. [Google Scholar] [CrossRef]
- Lucassen, W.; Geersing, G.J.; Erkens, P.M.; Reitsma, J.B.; Moons, K.G.; Büller, H.; van Weert, H.C. Clinical decision rules for excluding pulmonary embolism: A meta-analysis. Ann. Intern. Med. 2011, 155, 448–460. [Google Scholar] [CrossRef]
- Kirsch, B.; Aziz, M.; Kumar, S.; Burke, M.; Webster, T.; Immadi, A.; Sam, M.; Lal, A.; Estrada-Y-Martin, R.M.; Cherian, S.; et al. Wells score to predict pulmonary embolism in patients with coronavirus disease 2019. Am. J. Med. 2021, 134, 688–690. [Google Scholar] [CrossRef] [PubMed]




| Features | Global Population | PE (n = 17) | Non-PE (n = 33) | p |
|---|---|---|---|---|
| Age, years | 85.5 (80–90) | 83 (80–86) | 88 (81–91) | 0.264 |
| Sex (% male) | 26 (52.0) | 9 (52.9) | 17 (51.5) | 0.924 |
| Place of origin (%) | 0.475 | |||
| Home | 11 (22.0) | 5 (29.4) | 6 (18.2) | |
| Nursing home | 39 (78.0) | 12 (70.6) | 27 (81.8) | |
| BMI (n = 49) | 0.046 | |||
| Low weight | 5 (10.2) | 1 (5.9) | 4 (12.5) | |
| Normal weight | 25 (51.0) | 7 (41.2) | 18 (56.3) | |
| Overweight | 8 (16.3) | 2 (11.8) | 6 (18.8) | |
| Obesity | 11 (22.4) | 7 (41.2) | 4 (12.5) | |
| Time from clinical symptoms to admission, days | 8 (5–10) | 7 (4.5–9) | 8 (5–10) | 0.403 |
| Time from COVID diagnosis to CT scan, days | 14 (8–23) | 15 (10–23) | 12 (8–22) | 0.362 |
| Days of hospitalization | 14.5 (11–21) | 15 (13–28) | 14 (10–20) | 0.246 |
| Comorbidities, n (%) | ||||
| Oncological history | 10 (20.0) | 6 (35.3) | 4 (12.1) | 0.070 |
| DVT | 1 (2.0) | 1 (5.9) | 0 (0) | 0.340 |
| PE | 3 (6.0) | 0 (0) | 3 (9.1) | 0.542 |
| Trauma | 1 (2.0) | 0 (0) | 1 (3.0) | 1.000 |
| Neoplasia in palliative treatment | 2 (4.0) | 1 (5.9) | 1 (3.0) | 1.000 |
| Lower limbs pain | 2 (4.0) | 1 (5.9) | 1 (3.0) | 1.000 |
| PE symptoms | ||||
| Heart rate, beats/min (range) | 88 (80–100) | 96 (86–109) | 86 (76–96) | 0.015 |
| Tachycardia classification | 0.013 | |||
| 75–94 | 22 (44.0) | 5 (29.4) | 17 (51.5) | |
| >94 | 20 (40.0) | 11 (64.7) | 9 (27.3) | |
| DVT signs | 3 (6.0) | 2 (11.8) | 1 (3.0) | 0.264 |
| New DVT | 3 (6.0) | 2 (11.8) | 1 (3.0) | 0.264 |
| Pain/edema lower limbs | 4 (8.0) | 3 (17.6) | 1 (3.0) | 0.108 |
| Arterial embolic event | 0.108 | |||
| Lower limb ischemic events | 2 (4.0) | 1 (5.9) | 1 (3.0) | |
| Embolic stroke | 2 (4.0) | 2 (11.8) | 0 (0) | |
| Severity of the disease: CURB65 | 3 (2-3) | 2 (2-3) | 3 (2-3) | 0.431 |
| Geriatric assessment | ||||
| Dependency | 35 (70.0) | 10 (58.8) | 25 (75.8) | 0.216 |
| Frailty | 32 (64.0) | 10 (58.8) | 22 (66.7) | 0.584 |
| Polypharmacy | 34 (68.0) | 11 (64.7) | 23 (69.7) | 0.720 |
| Dementia | 20 (40.0) | 6 (35.3) | 14 (42.4) | 0.626 |
| Symptoms at hospitalization | ||||
| Fever | 22 (44.0) | 6 (35.3) | 16 (48.5) | 0.373 |
| Falls | 9 (18.0) | 5 (29.4) | 4 (12.1) | 0.242 |
| Dyspnea | 41 (82.0) | 13 (76.5) | 28 (84.8) | 0.468 |
| Loss of appetite | 12 (24.0) | 2 (11.8) | 10 (30.3) | 0.181 |
| Asthenia | 18 (36.0) | 3 (17.6) | 15 (45.5) | 0.052 |
| Delirium | 13 (26.0) | 3 (17.6) | 10 (30.3) | 0.499 |
| Cough | 11 (22.0) | 3 (17.6) | 8 (24.2) | 0.728 |
| Pneumonia | 1.000 | |||
| Unilateral | 12 (26.7) | 4 (25.0) | 8 (27.6) | |
| Bilateral | 33 (73.3) | 12 (75.0) | 21 (72.4) | |
| Medication | ||||
| Hydroxychloroquine | 37 (75.5) | 12 (75.0) | 25 (75.8) | 1.000 |
| Azithromycin | 27 (55.1) | 8 (50) | 19 (57.6) | 0.617 |
| Steroids | 24 (49) | 8 (50) | 16 (48.5) | 0.921 |
| PE prophylaxis | 47 (94.0) | 16 (94.1) | 31 (93.9) | 1.000 |
| Type of anticoagulation | 0.725 | |||
| Prophylactic dose | 35 (70.0) | 11 (64.7) | 24 (72.7) | |
| Full anticoagulation | 12 (24.0) | 5 (29.4) | 7 (21.2) | |
| Time of prophylaxis | 10 (8-14) | 10 (9-13) | 12 (6-15) | 0.623 |
| Mortality | 10 (20.0) | 3 (17.6) | 7 (21.2) | 1.000 |
| Characteristics | PE (n = 17) | Non-PE (n = 33) | p-Value |
|---|---|---|---|
| D-Dimer mg/L | 4.33 (2.40–7.17) | 1.39 (1.01–2.75) | <0.001 |
| NT-Pro-BNP pg/mL | 1273 (444–1908) | 1003 (501–2240) | 0.946 |
| Troponin ng/L | 40 (40–53) | 40 (40–55) | ND |
| CRP mg/L | 39.4 (21.0–248.0) | 62.5 (31.6–170.9) | 0.802 |
| Ferritin | 225 (159–463) | 243 (185–737) | 0.316 |
| Lymphocytes | 0.72 (0.55–1.20) | 0.75 (0.40–1.06) | 0.630 |
| DD mg/L | Sensitivity | Specificity | PPV | NPV | False Positives |
|---|---|---|---|---|---|
| 1.0 | 100% | 30.3% | 42.5% | 100% | 23% |
| 1.5 | 82.4% | 54.5% | 48.3% | 85.7% | 15% |
| 2.0 | 76.5% | 60.6% | 50% | 83.3% | 13% |
| 2.5 | 70.6% | 69.7% | 54.5% | 82.1% | 10% |
| 3.0 | 64.7% | 78.8% | 61.1% | 81.3% | 7% |
| 3.5 | 58.8% | 81.8% | 62.5% | 79.4% | 6% |
| 4.33 | 52.9% | 93.9% | 81.8% | 79.5% | 2% |
| Items | S | E | PPV | NPV |
|---|---|---|---|---|
| Wells score | 64.5 | 72.7 | 55.0 | 80.0 |
| Revised Geneva score | 82.4 | 60.6 | 51.0 | 87.0 |
| D-dimer | 52.9 | 93.9 | 81.8 | 79.5 |
| Wells score with D-dimer | 35.3 | 96.8 | 85.7 | 74.4 |
| Geneva score with D-dimer | 47.1 | 93.9 | 80 | 77.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quezada-Feijoo, M.; Ramos, M.; Lozano-Montoya, I.; Sarró, M.; Cabo Muiños, V.; Ayala, R.; Gómez-Pavón, F.J.; Toro, R. Elderly Population with COVID-19 and the Accuracy of Clinical Scales and D-Dimer for Pulmonary Embolism: The OCTA-COVID Study. J. Clin. Med. 2021, 10, 5433. https://doi.org/10.3390/jcm10225433
Quezada-Feijoo M, Ramos M, Lozano-Montoya I, Sarró M, Cabo Muiños V, Ayala R, Gómez-Pavón FJ, Toro R. Elderly Population with COVID-19 and the Accuracy of Clinical Scales and D-Dimer for Pulmonary Embolism: The OCTA-COVID Study. Journal of Clinical Medicine. 2021; 10(22):5433. https://doi.org/10.3390/jcm10225433
Chicago/Turabian StyleQuezada-Feijoo, Maribel, Mónica Ramos, Isabel Lozano-Montoya, Mónica Sarró, Verónica Cabo Muiños, Rocío Ayala, Francisco J. Gómez-Pavón, and Rocío Toro. 2021. "Elderly Population with COVID-19 and the Accuracy of Clinical Scales and D-Dimer for Pulmonary Embolism: The OCTA-COVID Study" Journal of Clinical Medicine 10, no. 22: 5433. https://doi.org/10.3390/jcm10225433
APA StyleQuezada-Feijoo, M., Ramos, M., Lozano-Montoya, I., Sarró, M., Cabo Muiños, V., Ayala, R., Gómez-Pavón, F. J., & Toro, R. (2021). Elderly Population with COVID-19 and the Accuracy of Clinical Scales and D-Dimer for Pulmonary Embolism: The OCTA-COVID Study. Journal of Clinical Medicine, 10(22), 5433. https://doi.org/10.3390/jcm10225433