Low-Intensity Resistance Training with Moderate Blood Flow Restriction Appears Safe and Increases Skeletal Muscle Strength and Size in Cardiovascular Surgery Patients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Reduction of Femoral Muscle Blood Flow by KAATSU
2.3. KAATSU RT Protocol
2.4. Evaluation of Clinical Data
- (1).
- Blood biochemistry: Fasting venous blood samples were obtained from the antecubital vein and analyzed for: hemoglobin A1 (HbA1c), albumin (Alb) brain natriuretic peptide (BNP), creatinine, creatine phosphokinase (CPK), high-sensitivity C-reactive protein (hsCRP), prothrombin time-international normalized ratio (PT-INR), and D-dimer (only for KAATSU RT group) using routine biochemical analysis performed in the hospital’s clinical laboratory.
- (2).
- Functional assessment: Maximum voluntary isometric contraction (MVIC) of the handgrip was determined with a factory-calibrated handgrip dynamometer (TKK 5401, TAKEI Scientific Instruments Co., Ltd., Tokyo, Japan). Each patient completed two trials and the higher value was used in the analysis. MVIC of the knee extensors was determined with a digital handheld dynamometer (μTas MT-1, ANIMA Co., Ltd., Tokyo, Japan) as described previously [1,30,31]. Patients were seated in a chair with the trunk vertical, the hip and knee joints flexed 90°, and arms folded across the chest. The load cell was affixed with a belt on the anterior aspect of the tibia just above the ankle so that the force was applied to the loadcell at 90°. Each patient completed two trials with 2 min of inter-trial rest. The higher of the two values was used in the analysis. Walking speed was computed as the time needed to walk 4 m at a habitual pace.
- (3).
- Body composition: A multi-frequency bioelectrical impedance analyzer (BIA) (InBody S10 Biospace devise, Biospace Co., Ltd., Korea/Model JMW140) was used according to the manufacturer’s recommendations [1,31]. Thirty impedance measurements were obtained at 1, 5, 50, 250, 500, and 1000 kHz over the right and left arms and legs and the trunk. The measurements were carried out while the subjects rested quietly in the supine position, with elbows extended and relaxed along the trunk. BIA-derived body composition metrics included body fat volume, % body fat, extracellular water (ECW), and total body water (TBW), and the ECW/TBW ratio was computed. Skeletal muscle mass index (SMI; appendicular skeletal muscle mass/height2, kg/m2) was measured as the sum of lean soft tissue of the two upper limbs and the two lower limbs. In this study, sarcopenia was defined according to the Asian Working Group for Sarcopenia (AWGS) [32] criteria (handgrip strength < 28 kgf for males and <18 kgf for females or walking speed ≤ 1.0 m/s; SMI < 7.0 kg/m2 for males and <5.7 kg/m2 for females).
- (4).
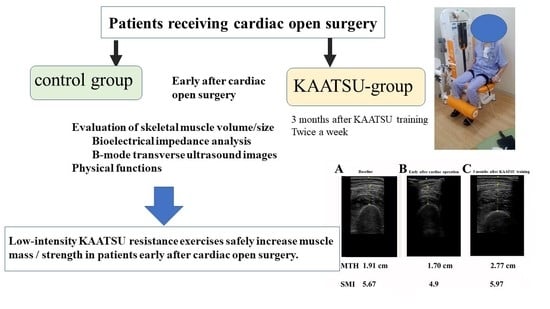
- Muscle size: Muscle thickness of thigh (MTH) was measured at the midpoint of the thigh length (between the greater trochanter and the lateral femoral condyle) with a real-time linear electronic scanner using a 10 MHz scanning head and ultrasound probe (L4-12t-RS Probe, GE Healthcare, Tokyo, Japan) and ultrasound (LOGIQ e, GE Healthcare, Tokyo, Japan) [1,30,31,33]. The scanning head was coated with a water-soluble transmission gel to provide acoustic contact without depressing the dermal surface. The subcutaneous adipose tissue-muscle interface and the muscle-bone interface were identified from the image. The perpendicular distance from the adipose tissue-muscle interface to muscle-bone interface was considered to represent MTH, measured in the supine position. Ink markers on the anterior and posterior thigh and posterior lower leg were used to ensure similar positioning over repeated MTH measurements. The measurement was performed twice on the right thigh and the average of the two values were used in the analysis. The test-retest reliability (Intraclass Correlation Coefficient (ICC), standard error of measurement (SEM), and minimal difference) was previously determined using the data of 9 older women measured twice within a few days for MTH (0.994, 0.28 cm, 0.79 cm).
- (5).
- Side effects: To verify the safety of KAATSU RT, all adverse and serious adverse events including deterioration of circulatory hemodynamics and hospitalizations were carefully monitored and recorded. We monitored the following potential side effects reported previously in conjunction with KAATSU RT: dizziness, subcutaneous hemorrhage, petechial hemorrhage, drowsiness, numbness, nausea, itchiness, etc. [21,22,23]. Furthermore, because surgery is a risk for deep vein thrombosis and KAATSU RT may increase the risk for developing new deep vein thrombosis in cardiac surgery patients, we carefully monitored patients’ lower limbs during the KAATSU RT. We also evaluated CPK and D-dimer after 3 months.
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Patients
3.2. Body Composition
3.3. Muscle Size
3.4. Physical Function
3.5. Correlation Analyses for Muscle Size and Function in the Control and KAATSU RT Group
3.6. Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RM | repetition maximum |
| EF | ejection fraction |
| Alb | Albumin |
| BNP | brain natriuretic peptide |
| CPK | creatinine phosphokinase |
| PT-INR | prothrombin time-international normalized ratio |
| BIA | bioelectrical impedance analyzer |
| ECW/TBW | extracellular water/total body water |
| SMI | skeletal muscle mass index |
| MTH | anterior thigh muscle thickness |
| DOMS | delayed-onset muscle soreness |
References
- Yasuda, T.; Nakajima, T.; Sawaguchi, T.; Nozawa, N.; Arakawa, T.; Takahashi, R.; Mizushima, Y.; Katayanagi, S.; Matsumoto, K.; Toyoda, S.; et al. Short physical performance battery for cardiovascular disease inpatients: Implications for critical factors and sarcopenia. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchikado, Y.; Ikeda, Y.; Ohishi, M. Current understanding of the role of frailty in cardiovascular disease. Circ. J. 2020, 84, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Sündermann, S.H.; Dademasch, A.; Seifert, B.; Rodriguez Cetina Biefer, H.; Emmert, M.Y.; Walther, T.; Jacobs, S.; Mohr, F.-W.; Falk, V.; Starck, C.T. Frailty is a predictor of short- and mid-term mortality after elective cardiac surgery independently of age. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 580–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiriya, Y.; Toshiaki, N.; Shibasaki, I.; Ogata, K.; Ogawa, H.; Takei, Y.; Tezuka, M.; Seki, M.; Kato, T.; Lefor, A.K.; et al. Sarcopenia assessed by the quantity and quality of skeletal muscle is a prognostic factor for patients undergoing cardiac surgery. Surg. Today 2020, 50, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Van Venrooij, L.M.; Verberne, H.J.; de Vos, R.; Borgmeijer-Hoelen, M.M.; van Leeuwen, P.A.; de Mol, B.A. Postoperative loss of skeletal muscle mass, complications and quality of life in patients undergoing cardiac surgery. Nutrition 2012, 28, 40–45. [Google Scholar] [CrossRef]
- Doyle, M.P.; Indraratna, P.; Tardo, D.T.; Peeceeyen, S.C.; E Peoples, G. Safety and efficacy of aerobic exercise commenced early after cardiac surgery: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 36–45. [Google Scholar] [CrossRef]
- Nohara, R.; Adachi, H.; Goto, Y.; Hsegawa, E.; Ishihara, S.; Itoh, H.; Kimura, Y.; Maehara, K.; Makita, S.; Matuso, H.; et al. Guidelines for rehabilitation in patients with cardiovascular disease (jcs 2012)—Digest version. Circ. J. 2014, 78, 2022–2093. [Google Scholar]
- Williams, M.A.; Haskell, W.L.; Ades, P.A.; Amsterdam, E.A.; Bittner, V.; Franklin, B.A.; Gulanick, M.; Laing, S.T.; Stewart, K.J. resistance exercise in individuals with and without cardiovascular disease 2007 update: A scientific statement from the American Heart Association Council on clinical cardiology and council on nutrition, physical activity, and metabolism. Circulation 2007, 116, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Pollock, M.L.; Franklin, B.A.; Balady, G.J.; Chaitman, B.L.; Fleg, J.L.; Fletcher, B.; Limacher, M.; Piña, I.L.; Stein, R.A.; Williams, M.; et al. AHA science advisory. resistance exercise in individuals with and without cardiovascular disease: Benefits, rationale, safety, and prescription: An advisory from the Committee on exercise, rehabilitation, and prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Cir-culation 2000, 101, 828–833. [Google Scholar]
- Leon, A.S.; Franklin, B.A.; Costa, F.; Balady, G.J.; Berra, K.A.; Stewart, K.J.; Thompson, P.D.; Williams, M.A.; Lauer, M.S. American Heart Association; et.al. Cardiac rehabilitation and secondary prevention of coronary heart disease: An American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2005, 111, 369–376. [Google Scholar]
- Kraemer, W.J.; Adams, K.; Cafarelli, E.; Dudley, G.A.; Dooly, C.; Feigenbaum, M.S.; Fleck, S.J.; Franklin, B.; Fry, A.C.; Hoffman, J.R.; et al. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2002, 34, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y.; Ishii, N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, T.; Kearns, C.F.; Sato, Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J. Appl. Physiol. 2006, 100, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Abe, T.; Drummond, M.J.; Cadenas, J.G.; Dreyer, H.C.; Sato, Y.; Volpi, E.; Rasmussen, B.B. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J. Appl. Physiol. 2007, 103, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Wernbom, M.; Augustsson, J.; Raastad, T. Ischemic strength training: A low-load alternative to heavy resistance exercise? Scand. J. Med. Sci. Sports 2008, 18, 401–416. [Google Scholar] [CrossRef]
- Baker, B.S.; Stannard, M.S.; Duren, D.L.; Cook, J.L.; Stannard, J.P. Does blood flow restriction therapy in patients older than age 50 result in muscle hypertrophy, increased strength, or greater physical function? A systematic review. Clin. Orthop. Relat. Res. 2020, 478, 593–606. [Google Scholar] [CrossRef]
- Yasuda, T.; Brechue, W.F.; Fujita, T.; Shirakawa, J.; Sato, Y.; Abe, T. Muscle activation during low-intensity muscle contractions with restricted blood flow. J. Sports Sci. 2009, 27, 479–489. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Fukuda, T.; Iida, H.; Imuta, H.; Sato, Y.; Yamasoba, T.; Nakajima, T. Effects of low-intensity, elastic band resistance exercise combined with blood flow restriction on muscle activation. Scand. J. Med. Sci. Sports 2012, 24, 55–61. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Fahs, C.A.; Wilson, J.M.; Bemben, M.G. Blood flow restriction: The metabolite/volume threshold theory. Med. Hypotheses 2011, 77, 748–752. [Google Scholar] [CrossRef]
- Ishizaka, H.; Uematsu, A.; Mizushima, Y.; Nozawa, N.; Katayanagi, S.; Matsumoto, K.; Nishikawa, K.; Takahashi, R.; Arakawa, T.; Sawaguchi, T.; et al. Blood flow restriction increases the neural activation of the knee extensors during very low-intensity leg extension exercise in cardiovascular patients: A pilot study. J. Clin. Med. 2019, 8, 1252. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Kurano, M.; Iida, H.; Takano, H.; Oonuma, H.; Morita, T.; Meguro, K.; Sato, Y.; Nagata, T. Use and safety of KAATSU training: Results of a national survey. Int. J. Kaatsu Train. Res. 2006, 2, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Morita, T.; Sato, Y. Key considerations when conducting KAATSU training. Int. J. KAATSU Train. Res. 2011, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, T.; Meguro, M.; Sato, Y.; Nakajima, T. Use and safety of KAATSU training: Results of a national survey in 2016. Int. J. KAATSU Train. Res. 2017, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Patel, B.H.; Kym, C.; Nwachukwu, B.U.; Beletksy, A.; Forsythe, B.; Chahla, J. Perioperative blood flow restriction rehabilitation in patients undergoing acl reconstruction: A systematic review. Orthop. J. Sports Med. 2020, 8, 2325967120906822. [Google Scholar] [CrossRef]
- Nakajima, T.; Kurano, M.; Sakagami, F.; Iida, H.; Fukumura, K.; Fukuda, T.; Takano, H.; Madarame, H.; Yasuda, T.; Nagata, T.; et al. Effects of low-in-tensity KAATSU resistance training on skeletal muscle size/strength and endurance capacity in patients with ischemic heart disease. Int. J. Kaatsu Train. Res. 2010, 6, 1–7. [Google Scholar] [CrossRef]
- Takano, H.; Morita, T.; Iida, H.; Asada, K.-I.; Kato, M.; Uno, K.; Hirose, K.; Matsumoto, A.; Takenaka, K.; Hirata, Y.; et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 95, 65–73. [Google Scholar] [CrossRef]
- Iida, H.; Kurano, M.; Takano, H.; Kubota, N.; Morita, T.; Meguro, K.; Sato, Y.; Abe, T.; Yamazaki, Y.; Uno, K.; et al. Hemodynamic and neurohumoral responses to the restriction of femoral blood flow by KAATSU in healthy subjects. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 100, 275–285. [Google Scholar] [CrossRef]
- Nakajima, T.; Iida, H.; Kurano, M.; Takano, H.; Morita, T.; Meguro, K.; Sato, Y.; Yamazaki, Y.; Kawashima, S.; Ohshima, H.; et al. Hemodynamic responses to simulated weightlessness of 24-h head-down bed rest and KAATSU blood flow restriction. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 104, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Nakajima, T. Short physical performance battery for middle-aged and older adult cardiovascular disease patients: Implication for strength tests and lower extremity morphological evaluation. J. Physiol. Ther. Sci. 2017, 29, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Hirose, S.; Nakajima, T.; Nozawa, N.; Katayanagi, S.; Ishizaka, H.; Mizushima, Y.; Matsumoto, K.; Nishikawa, K.; Toyama, Y.; Takahashi, R.; et al. Phase angle as an indicator of sarcopenia, malnutrition, and cachexia in inpatients with cardiovascular diseases. J. Clin. Med. 2020, 9, 2554. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Toyoda, S.; Inoue, T.; Nakajima, T. Muscle thickness of anterior mid-thigh in hospitalized patients: Comparison of supine and standing postures. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100063. [Google Scholar] [CrossRef]
- Madarame, H.; Kurano, M.; Takano, H.; Iida, H.; Sato, Y.; Ohshima, H.; Abe, T.; Ishii, N.; Morita, T.; Nakajima, T. Effects of low-intensity resistance exercise with blood flow restriction on coagulation system in healthy subjects. Clin. Physiol. Funct. Imaging 2010, 30, 210–213. [Google Scholar] [CrossRef]
- Umbel, J.D.; Hoffman, R.L.; Dearth, D.J.; Chleboun, G.S.; Manini, T.M.; Clark, B.C. Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 107, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Spranger, M.D.; Krishnan, A.C.; Levy, P.D.; O’Leary, D.S.; Smith, S.A. Blood flow restriction training and the exercise pressor reflex: A call for concern. Am. J. Physiol. Circ. Physiol. 2015, 309, H1440–H1452. [Google Scholar] [CrossRef]
- Cristina-Oliveira, M.; Meireles, K.; Spranger, M.D.; O’Leary, D.S.; Roschel, H.; Peçanha, T. Clinical safety of blood flow-restricted training? A comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. Am. J. Physiol. Circ. Physiol. 2020, 318, H90–H109. [Google Scholar] [CrossRef]
- Barak, N.; Wall-Alonso, E.; Cheng, A.; Sitrin, M.D. Use of bioelectrical impedance analysis to predict energy expenditure of hospitalized patients receiving nutrition support. J. Parenter. Enter. Nutr. 2003, 27, 43–46. [Google Scholar] [CrossRef]
- Bloch, S.A.; Lee, J.Y.; Wort, S.J.; Polkey, M.I.; Kemp, P.R.; Griffiths, M.J. Sustained elevation of circulating growth and differ-entiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit. Care. Med. 2013, 1, 82–89. [Google Scholar]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Number | Control (n = 10) | KAATSU RT (n = 11) | p value | Cohen’s d |
|---|---|---|---|---|
| Male: Female | 9: 1 | 9: 2 | ||
| Age, year | 66 ± 8.7 | 57 ± 12.2 | 0.08 | 0.82 |
| Body mass, kg | 62.7 ± 8.3 | 61.5 ± 9.4 | 0.80 | 0.11 |
| BMI, kg/m2 | 23.1 ± 2.3 | 22.1 ± 3.6 | 0.45 | 0.31 |
| Height, cm | 165.2 ± 7.4 | 167 ± 12 | 0.34 | 0.19 |
| Risk factors, number | ||||
| Hypertension | 7 | 8 | 0.63 | |
| Diabetes | 4 | 3 | 0.44 | |
| Dyslipidemia | 2 | 0 | 0.21 | |
| Smoking | 1 | 1 | 0.74 | |
| Hemodialysis | 0 | 0 | ||
| Atrial fibrillation | 4 | 4 | 0.61 | |
| NYHA I–IV, number | I: 0 | I: 0 | ||
| II: 9 | II: 9 | 0.54 | ||
| III: 1 | III: 2 | 0.54 | ||
| IV: 0 | IV: 0 | |||
| Disease, number | Severe AS: 4 | Severe AS: 3 | 0.44 | |
| Severe AR: 1 | Severe AR: 4 | 0.18 | ||
| Severe MS: 1 | Severe MS: 1 | 0.74 | ||
| Severe MR: 3 | Severe MR: 3 | 0.63 | ||
| A P: 1 | ||||
| Cardiovascular surgery, number | AVR: 5 | AVR: 6 | 0.59 | |
| MVR: 1 | MVR: 1 | 0.74 | ||
| MVP: 3 | MVP: 3 | 0.63 | ||
| other | CABG: 1 | Bentall: 1 | ||
| Creatinine, mg/dL | 0.94 ± 0.2 | 1.06 ± 0.24 | 0.25 | 0.54 |
| Hb, g/dL | 13.6 ± 1.5 | 13.1 ± 2.0 | 0.51 | 0.31 |
| Alb, g/dL | 4.2 ± 0.2 | 3.9 ± 0.6 | 0.06 | 0.89 |
| HbA1c, % | 6.0 ± 0.4 | 5.8 ± 0.6 | 0.33 | 0.44 |
| BNP, pg/ml | 172 ± 138 | 303 ± 262 | 0.14 | 0.69 |
| EF, % | 59 ± 11 | 54 ± 16 | 0.31 | 0.45 |
| Control | KAATSU RT | |||||
|---|---|---|---|---|---|---|
| Baseline | Early after Cardiac Surgery | After 3 Months | Baseline | Early after Cardiac Surgery | After 3 Months | |
| Body mass, kg | 62.7 (8.3) | 60.6 (8.2) | 61.4 (8.0) | 61.5 (9.4) | 58.5 (7.9) * | 62.2 (8.1) # |
| BMI, kg/m2 | 23.1 (2.3) | 22.2 (2.4) | 24.1 (5.0) | 22.1 (3.6) | 20.9 (3) | 23 (3.2) |
| Body fat volume, kg | 17.5 (3.8) | 16.4 (4) | 16.9 (3.6) | 16.4 (6.5) | 14.9 (6) | 16.4 (5.9) |
| Body fat percentage, % | 25.4 (7.5) | 27.1 (6.2) | 25.9 (7) | 25 (6.5) | 25.4 (11) | 25 (10) |
| ECW/TBW | 0.39 (0.012) | 0.39 (0.007) | 0.40 (0.015) | 0.39 (0.007) | 0.40 (0.005) | 0.39 (0.004) |
| MTH, cm | 2.8 (0.9) | 2.6 (1.0) | 2.8 (0.9) | 2.5 (0.5) | 2.1 (0.4) * | 3.0 (0.5) *,# |
| SMI, kg/m2 | 7.0 (0.9) | 6.7 (0.9) | 7.0 (1.0) | 6.9 (1.0) | 6.5 (0.8) * | 7.3 (0.8) *,# |
| Walking speed, m/s | 1.1 (0.2) | 1.0 (0.2) | 1.2 (0.1) | 1.1 (0.2) | 1.2 (0.2) | 1.39 (0.2) *,# |
| Handgrip, kgf | 31.3 (7.4) | 28.3 (8.2) | 30.7 (6.7) | 30.3 (7.5) | 29.2 (5.2) | 33.9 (8.5) |
| Knee extensor, kgf | 33.5 (10.5) | 28 (10.4) | 31.7 (7.48) | 30.5 (11.2) | 29.2 (5.2) | 41.8 (15.1) *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, H.; Nakajima, T.; Shibasaki, I.; Nasuno, T.; Kaneda, H.; Katayanagi, S.; Ishizaka, H.; Mizushima, Y.; Uematsu, A.; Yasuda, T.; et al. Low-Intensity Resistance Training with Moderate Blood Flow Restriction Appears Safe and Increases Skeletal Muscle Strength and Size in Cardiovascular Surgery Patients: A Pilot Study. J. Clin. Med. 2021, 10, 547. https://doi.org/10.3390/jcm10030547
Ogawa H, Nakajima T, Shibasaki I, Nasuno T, Kaneda H, Katayanagi S, Ishizaka H, Mizushima Y, Uematsu A, Yasuda T, et al. Low-Intensity Resistance Training with Moderate Blood Flow Restriction Appears Safe and Increases Skeletal Muscle Strength and Size in Cardiovascular Surgery Patients: A Pilot Study. Journal of Clinical Medicine. 2021; 10(3):547. https://doi.org/10.3390/jcm10030547
Chicago/Turabian StyleOgawa, Hironaga, Toshiaki Nakajima, Ikuko Shibasaki, Takahisa Nasuno, Hiroyuki Kaneda, Satoshi Katayanagi, Hayato Ishizaka, Yuta Mizushima, Azusa Uematsu, Tomohiro Yasuda, and et al. 2021. "Low-Intensity Resistance Training with Moderate Blood Flow Restriction Appears Safe and Increases Skeletal Muscle Strength and Size in Cardiovascular Surgery Patients: A Pilot Study" Journal of Clinical Medicine 10, no. 3: 547. https://doi.org/10.3390/jcm10030547
APA StyleOgawa, H., Nakajima, T., Shibasaki, I., Nasuno, T., Kaneda, H., Katayanagi, S., Ishizaka, H., Mizushima, Y., Uematsu, A., Yasuda, T., Yagi, H., Toyoda, S., Hortobágyi, T., Mizushima, T., Inoue, T., & Fukuda, H. (2021). Low-Intensity Resistance Training with Moderate Blood Flow Restriction Appears Safe and Increases Skeletal Muscle Strength and Size in Cardiovascular Surgery Patients: A Pilot Study. Journal of Clinical Medicine, 10(3), 547. https://doi.org/10.3390/jcm10030547