A Retrospective Analysis of Characteristic Features of Responders and Impaired Patients to a Single Injection of Pure Platelet-Rich Plasma in Knee Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
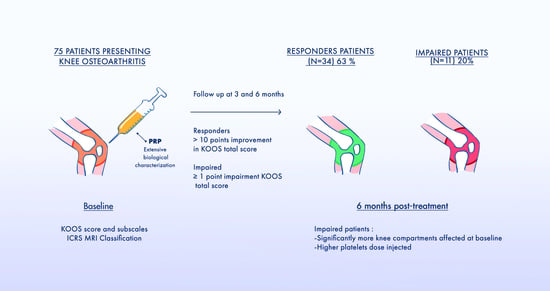
2.2. Evaluation Tools and Follow-Up
2.3. Platelet-Rich Plasma Preparation
2.4. Biological Parameter Quantification
2.4.1. Blood and PRP Cell Counting
2.4.2. Growth Factors Release Measurement
2.4.3. Microbiological Assay
2.5. Injection
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Biological Characteristics of PRP Injected
3.3. Evolution of the KOOS Score
3.4. Comparison of Responders and Impaired Patients Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Hussain, S.; Neilly, D.; Baliga, S.; Patil, S.; Meek, R. Knee osteoarthritis: A review of management options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Beaumont, G.; Roman-Blas, J.A.; Bruyère, O.; Cooper, C.; Kanis, J.; Maggi, S.; Rizzoli, R.; Reginster, J.-Y. Clinical settings in knee osteoarthritis: Pathophysiology guides treatment. Maturitas 2017, 96, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, J.; Fitzpatrick, J.; Perera, N.K.P.; Orchard, J. Osteoarthritis-a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet. Disord. 2019, 20, 151. [Google Scholar] [CrossRef]
- Kompel, A.; Roemer, F.; Murakami, A.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Safe as We Thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Torrero, J.I.; Martínez, C. New developments in the treatment of osteoarthritis-focus on biologic agents. Open Access. Rheumatol. 2015, 7, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Rachul, C.; Rasko, J.E.J.; Caulfield, T. Implicit hype? Representations of platelet rich plasma in the news media. PLoS ONE 2017, 12, e0182496. [Google Scholar]
- Gupta, S.; Paliczak, A.; Delgado, D. Evidence-based indications of platelet-rich plasma therapy. Expert. Rev. Hematol. 2021, 14, 97–108. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Halper, J. Advances in the use of growth factors for treatment of disorders of soft tissues. Adv. Exp. Med. Biol. 2014, 802, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.I.; Whitney, K.; Evans, T.; LaPrade, R.F. Platelet-Rich Plasma and Cartilage Repair. Curr. Rev. Musculoskelet. Med. 2018, 11, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; Laprade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Jt. Surg. Am. 2017, 99, 1769–1779. [Google Scholar] [CrossRef]
- Magalon, J.; Brandin, T.; Francois, P.; Degioanni, C.; De Maria, L.; Grimaud, F.; Veran, J.; Dignat-George, F.; Sabatier, F. Technical and biological review of authorized medical devices for platelets-rich plasma preparation in the field of regenerative medicine. Platelets 2020, 32, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: A call for PRP standardization. Reg. Anesth. Pain Med. 2019, 44, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: Implications for intra-articular injury and therapy. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2020, 49, 249–260. [Google Scholar] [CrossRef]
- Kuroki, H.; Nakagawa, Y.; Mori, K.; Kobayashi, M.; Yasura, K.; Okamoto, Y.; Suzuki, T.; Nishitani, K.; Nakamura, T. Ultrasound properties of articular cartilage in the tibio-femoral joint in knee osteoarthritis: Relation to clinical assessment (International Cartilage Repair Society grade). Arthritis Res. Ther. 2008, 10, R78. [Google Scholar] [CrossRef] [Green Version]
- Graiet, H.; Lokchine, A.; Francois, P.; Velier, M.; Grimaud, F.; Loyens, M.; Berda-Haddad, Y.; Veran, J.; Dignat-George, F.; Sabatier, F.; et al. Use of platelet-rich plasma in regenerative medicine: Technical tools for correct quality control. BMJ Open Sport Exerc. Med. 2018, 4, e000442. [Google Scholar] [CrossRef] [Green Version]
- Guillibert, C.; Charpin, C.; Raffray, M.; Benmenni, A.; Dehaut, F.-X.; El Ghobeira, G.; Giorgi, R.; Magalon, J.; Arniaud, D. Single Injection of High Volume of Autologous Pure PRP Provides a Significant Improvement in Knee Osteoarthritis: A Prospective Routine Care Study. Int. J. Mol. Sci. 2019, 20, 1327. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, A.K.; Davis, K.W.; Ross, A.; Rosas, H.G. Fundamentals of Joint Injection. AJR Am. J. Roentgenol. 2016, 207, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Hua, S.; Yang, T.; Ma, J.; Yu, W.; Chen, X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp. Ther. Med. 2018, 15, 3096–3102. [Google Scholar] [CrossRef] [Green Version]
- Everts, P.A.; Malanga, G.A.; Paul, R.V.; Rothenberg, J.B.; Stephens, N.; Mautner, K.R. Assessing clinical implications and perspectives of the pathophysiological effects of erythrocytes and plasma free hemoglobin in autologous biologics for use in musculoskeletal regenerative medicine therapies. A review. Regen. Ther. 2019, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, K.D.; El Bitar, Y.; Siewert, K.; Scaife, S.L.; El-Amin, S.; Saleh, K.J. Correlation of WOMAC and KOOS scores to tibiofemoral cartilage loss on plain radiography and 3 Tesla MRI: Data from the osteoarthritis initiative. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Rodeo, S.; Bhutani, N.; Goodrich, L.R.; Huard, J.; Irrgang, J.; Laprade, R.F.; Lattermann, C.; Lu, Y.; Mandelbaum, B.; et al. Optimizing Clinical Use of Biologics in Orthopaedic Surgery: Consensus Recommendations From the 2018 AAOS/NIH U-13 Conference. J. Am. Acad. Orthop. Surg. 2019, 27, e50–e63. [Google Scholar] [CrossRef]
- Murray, I.R.; Geeslin, A.G.; Goudie, E.B.; Petrigliano, F.A.; LaPrade, R.F. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): Platelet-Rich Plasma and Mesenchymal Stem Cells. J. Bone Jt. Surg. Am. 2017, 99, 809–819. [Google Scholar] [CrossRef]
- Buttarello, M.; Plebani, M. Automated blood cell counts: State of the art. Am. J. Clin. Pathol. 2008, 130, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straum, O.K. The optimal platelet concentration in platelet-rich plasma for proliferation of human cells in vitro-diversity, biases, and possible basic experimental principles for further research in the field: A review. PeerJ 2020, 8, e10303. [Google Scholar] [CrossRef]
- Graziani, F.; Ivanovski, S.; Cei, S.; Ducci, F.; Tonetti, M.; Gabriele, M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral. Implants Res. 2006, 17, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabestari, M.; Shabestari, Y.R.; Landin, M.A.; Pepaj, M.; Cleland, T.P.; Reseland, J.E.; Eriksen, E.F. Altered protein levels in bone marrow lesions of hip osteoarthritis: Analysis by proteomics and multiplex immunoassays. Int. J. Rheum. Dis. 2020, 23, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 3971. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.L.; Magalon, J.; Jouve, E.; Bornet, C.E.; Mattei, J.C.; Chagnaud, C.; Rochwerger, A.; Veran, J.; Sabatier, F. Growth Factors Levels Determine Efficacy of Platelets Rich Plasma Injection in Knee Osteoarthritis: A Randomized Double Blind Noninferiority Trial Compared With Viscosupplementation. Arthroscopy 2018, 34, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.C.; van de Loo, F.A.; van Beuningen, H.M.; Sime, P.; van Lent, P.; van der Kraan, P.; Richards, C.; Berg, W.V.D. Overexpression of active TGF-beta-1 in the murine knee joint: Evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthr. Cartil. 2001, 9, 128–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaney Davidson, E.N.; van der Kraan, P.M.; van den Berg, W.B. TGF-beta and osteoarthritis. Osteoarthr. Cartil. 2007, 15, 597–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaPrade, R.F.; Dragoo, J.L.; Koh, J.L.; Murray, I.R.; Geeslin, A.G.; Chu, C.R. AAOS Research Symposium Updates and Consensus: Biologic Treatment of Orthopaedic Injuries. J. Am. Acad. Orthop. Surg. 2016, 24, e62. [Google Scholar] [CrossRef]
- Li, H.; Hicks, J.J.; Wang, L.; Oyster, N.; Philippon, M.J.; Hurwitz, S.; Hogan, M.V.; Huard, J. Customized platelet-rich plasma with transforming growth factor β1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials 2016, 87, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Robertson, J.; Jones, A.C.; Dieppe, P.A.; Doherty, M. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2008, 67, 1716–1723. [Google Scholar] [CrossRef]


| Biological Characteristics | Mean ± SD | Min-Max | Coefficient of Variation (%) |
|---|---|---|---|
| Injected Volume (mL) | 6.77 ± 1.43 | 5.00–9.00 | 21.04 |
| Increase factor in platelets | 1.82 ± 0.30 | 0.86–2.65 | 16.35 |
| Increase factor in leucocytes | 0.16 ± 0.12 | 0.003–0.540 | 76.20 |
| Recovery rate in platelets (%) | 85.15 ± 10.05 | 62.30–103.00 | 11.80 |
| Sterility (%) | 100 | 100 | 0 |
| Relative composition | |||
| Platelets (%) | 95.46 ± 2.01 | 89.40–98.67 | 2.11 |
| Leucocytes (%) | 0.19 ± 0.14 | 0.01–0.58 | 73.27 |
| Red blood cells (%) | 4.35 ± 1.96 | 1.31–10.07 | 45.03 |
| Absolute composition | |||
| Platelets dose injected (billion, 109) | 2.87 ± 0.88 | 0.91–4.45 | 30.63 |
| Leucocytes dose injected (million, 106) | 6.06 ± 5.44 | 0.10–27.65 | 52.57 |
| Red blood cells dose injected (billion, 109) | 0.13 ± 0.07 | 0.05–0.33 | 53.48 |
| Platelets relative parameters | |||
| Immature Platelet Fraction (%) | 3.44 ± 1.83 | 1.00–9.00 | 53.20 |
| Immature platelets (billion, 109) | 0.10 ± 0.06 | 0.02–0.27 | 52.57 |
| Mean platelets volume (fL) | 10.14 ± 0.68 | 8.80–12.00 | 53.48 |
| Growth factors * | |||
| Inflammatory | |||
| TNF-α (pg) | 141.6 ± 68.56 | 35.75–387.8 | 48.41 |
| IL-6 (pg) | Not detectable | Not detectable | Not detectable |
| IFN-g (pg) | Not detectable | Not detectable | Not detectable |
| Anti inflammatory | |||
| IL-1Ra (pg) | 164.0 ± 267.9 | 0.0–1125.0 | 163.34 |
| IL-10 (pg) | Not detectable | Not detectable | Not detectable |
| Regenerative | |||
| PDGF AB BB (fg) | 228.4 ± 100.4 | 69.6 ± 469.3 | 43.94 |
| VEGF (pg) | 451.6 ± 421.6 | 0.0–1476 | 93.34 |
| EGF (pg) | 1231 ± 721.0 | 138.8–4334 | 58.59 |
| FGF-2 (pg) ** | 1157 ± 1173 | 0.0–4164.0 | 101.34 |
| TGF-β1 (fg) | 361.2 ± 107.6 | 154.3 ± 675.1 | 29.79 |
| Responders (n = 34) | Impaired (n = 11) | p-Value | |
|---|---|---|---|
| Age | 61.00 ± 11.00 | 66.73 ± 7.00 | 0.09 |
| Sex Ratio (H/F) | 12/22 | 2/9 | 0.46 |
| Body Mass Index (kg/m2) | 26.36 ± 3.60 | 27.24 ± 2.98 | 0.44 |
| Previous HA injection: n (%) | 28 (82.0) | 8 (82.0) | 1 |
| Previous PRP injection: n (%) | 8 (23.5) | 4 (36.4) | 0.06 |
| Volume injected | 6.50 ± 1.53 | 6.36 ±1.57 | 0.40 |
| Baseline KOOS score | 35.97 ± 16.42 | 47.55 ± 14.07 | 0.02 |
| Pain | 47.59 ± 20.89 | 52.36 ± 16.77 | 0.54 |
| Symptoms | 48.74 ± 22.51 | 60.09 ± 16.80 | 0.15 |
| ADL | 48.29 ± 21.29 | 16.09 ± 14.62 | 0.27 |
| Sport | 18.85 ± 20.62 | 35.50 ±14.23 | 0.01 |
| QOL | 16.82 ± 14.05 | 31.90 ± 25.44 | 0.03 |
| Kellgren and Lawrence scale (%) | |||
| I | 17.7% | 0% | |
| II | 17.7% | 18.2% | <0.0001 |
| III | 64.7% | 36.4% | |
| IV | 0% | 45.4% | |
| MRI characteristics according ICRS classification | |||
| Proportion of patient presenting: | |||
| One compartment affected | 11.5% | 3.8% | 0.01 |
| Two compartments affected | 49.1% | 38.5% | |
| Three compartments affected | 39.3% | 57.7% | |
| Proportion of patients with at least one compart-ment presenting grade IV OA: | 46.7% | 73.0% | 0.17 |
| Pain at the injection (VAS) | 27.88 ± 27.36 | 32.73 ± 24.12 | 0.55 |
| Biological Characteristics | Responders (n = 34) | Impaired (n = 11) | p |
|---|---|---|---|
| Injected Volume (mL) | 6.51 ± 1.53 | 6.36 ± 1.57 | 0.40 |
| Increase factor of platelets | 1.76 ± 0.33 | 1.87 ± 0.11 | 0.08 |
| Increase factor of leukocytes | 0.13 ± 0.11 | 0.19 ± 0.15 | 0.11 |
| Relative composition | |||
| Platelets (%) | 95.42 ± 1.76 | 95.87 ± 1.89 | 0.31 |
| Leukocytes (%) | 0.16 ± 0.12 | 0.20 ± 0.19 | 0.49 |
| Red blood cells (%) | 4.41 ± 1.74 | 3.93 ± 1.74 | 0.32 |
| Absolute composition | |||
| Platelets (109) | 2.60 ± 0.91 | 3.28 ± 0.87 | 0.01 |
| Leukocytes (106) | 4.80 ± 5.27 | 7.03 ± 6.12 | 0.10 |
| Red blood cells (109) | 0.108 ± 0.052 | 0.123 ± 0.087 | 0.42 |
| Platelets relative parameters | |||
| Immature Platelet Fraction (%) | 3.33 ± 1.79 | 2.87 ± 1.72 | 0.19 |
| Immature platelets (109) | 0.08 ± 0.05 | 0.09 ± 0.07 | 0.28 |
| Mean platelet volume (fL) | 10.21 ± 0.70 | 9.80 ± 0.72 | 0.08 |
| Growth factors | n = 25 | n = 8 | |
| Inflammatory | |||
| TNF-α (pg) | 123.4 ± 51.3 | 162.0 ± 96.3 | 0.22 |
| IL-6 | Not detectable | Not detectable | Not detectable |
| IFN-g | Not detectable | Not detectable | Not detectable |
| Anti inflammatory | |||
| IL-1Ra (pg) | 113.9 ± 179.9 | 378.3 ± 451.5 | 0.05 |
| IL-10 (pg) | Not detectable | Not detectable | Not detectable |
| Regenerative | |||
| PDGF AB BB (fg) | 216.6 ± 81.3 | 241.3 ± 131.9 | 0.29 |
| VEGF (pg) | 401.8 ± 345.1 | 803.5 ± 445.0 | 0.008 |
| EGF (pg) | 1068.0 ± 607.4 | 1453.0 ± 368.9 | 0.05 |
| FGF-2 (pg) | 1026.0 ± 746.5 | 1200 ± 1726 | 0.32 |
| TGF-β1 (fg) | 354.8 ± 103.5 | 397.1 ± 118.7 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bec, C.; Rousset, A.; Brandin, T.; François, P.; Rabarimeriarijaona, S.; Dumoulin, C.; Heleu, G.; Grimaud, F.; Veran, J.; Magalon, G.; et al. A Retrospective Analysis of Characteristic Features of Responders and Impaired Patients to a Single Injection of Pure Platelet-Rich Plasma in Knee Osteoarthritis. J. Clin. Med. 2021, 10, 1748. https://doi.org/10.3390/jcm10081748
Bec C, Rousset A, Brandin T, François P, Rabarimeriarijaona S, Dumoulin C, Heleu G, Grimaud F, Veran J, Magalon G, et al. A Retrospective Analysis of Characteristic Features of Responders and Impaired Patients to a Single Injection of Pure Platelet-Rich Plasma in Knee Osteoarthritis. Journal of Clinical Medicine. 2021; 10(8):1748. https://doi.org/10.3390/jcm10081748
Chicago/Turabian StyleBec, Cécilia, Axelle Rousset, Thibault Brandin, Pauline François, Sitraka Rabarimeriarijaona, Chloé Dumoulin, Gaëlle Heleu, Fanny Grimaud, Julie Veran, Guy Magalon, and et al. 2021. "A Retrospective Analysis of Characteristic Features of Responders and Impaired Patients to a Single Injection of Pure Platelet-Rich Plasma in Knee Osteoarthritis" Journal of Clinical Medicine 10, no. 8: 1748. https://doi.org/10.3390/jcm10081748
APA StyleBec, C., Rousset, A., Brandin, T., François, P., Rabarimeriarijaona, S., Dumoulin, C., Heleu, G., Grimaud, F., Veran, J., Magalon, G., Dignat-George, F., Sabatier, F., Louis, M. -L., & Magalon, J. (2021). A Retrospective Analysis of Characteristic Features of Responders and Impaired Patients to a Single Injection of Pure Platelet-Rich Plasma in Knee Osteoarthritis. Journal of Clinical Medicine, 10(8), 1748. https://doi.org/10.3390/jcm10081748