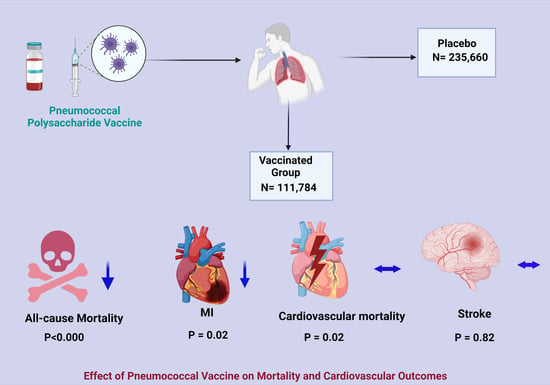
Effect of Pneumococcal Vaccine on Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Outcomes
2.3. Inclusion Criteria
- Studies with patients of age ≥ 18.
- Studies including intervention and comparison groups where the intervention group was considered as patients who receive the pneumococcal vaccine (PV), while the comparison group was patients that were either unvaccinated or received a placebo.
- Studies should report on the desired outcomes, i.e., all-cause mortality, risk of MI, stroke, and cardiovascular (CV) mortality.
- Eligible study designs included RCTs, prospective and retrospective cohort studies.
2.4. Exclusion Criteria
- Animal studies, abstracts, editorials, commentaries, systematic reviews, single patient case studies, letters, and studies with insufficient data were excluded.
- Studies where the pneumococcal and influenza vaccines were compared instead of pneumococcal alone with placebo or unvaccinated groups were also excluded.
2.5. Data Extraction and Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Studies Characteristics
3.3. Baseline Patient Demographics
3.4. Meta-Analysis of Study Outcomes
3.4.1. Primary Outcomes—All-Cause Mortality
3.4.2. Secondary Outcomes—Cardiovascular Mortality, Acute MI, and Stroke
3.4.3. Meta-Regression
3.4.4. Publication Bias
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Disease (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 April 2022).
- Jackson, M.L.; Nelson, J.C.; Jackson, L.A. Risk factors for community-acquired pneumonia in immunocompetent seniors. J. Am. Geriatr. Soc. 2009, 57, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.P.; O’Brien, K.L.; Benin, A.L.; McCoy, S.I.; Donaldson, C.M.; Reid, R.; Schuchat, A.; Zell, E.R.; Hochman, M.; Santosham, M.; et al. Risk factors for invasive pneumococcal disease among Navajo adults. Am. J. Epidemiol. 2007, 166, 1080–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Gomez, N.; Anzueto, A.; Waterer, G.W.; Restrepo, M.I.; Mortensen, E.M. Pneumonia: An arrhythmogenic disease? Am. J. Med. 2013, 126, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrales-Medina, V.F.; Musher, D.M.; Shachkina, S.; Chirinos, J.A. Acute pneumonia and the cardiovascular system. Lancet 2013, 381, 496–505. [Google Scholar] [CrossRef]
- Africano, H.; Serrano-Mayorga, C.; Ramirez-Valbuena, P.; Bustos, I.; Bastidas, A.; Vargas, H.; Gómez, S.; Rodriguez, A.; Orihuela, C.; Reyes, L. Major Adverse Cardiovascular Events during Invasive Pneumococcal Disease Are Serotype Dependent. Clin. Infect. Dis. 2020, 72, e711–e719. [Google Scholar] [CrossRef]
- Barrios, V.; Escobar, C. Vaccination in Patients with Heart Disease. How Long Should We Wait? Rev. Española De Cardiol. (Engl. Ed.) 2019, 72, 515. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Suh, K.N.; Rose, G.; Chirinos, J.A.; Doucette, S.; Cameron, D.W.; Fergusson, D.A. Cardiac complications in patients with community-acquired pneumonia: A systematic review and meta-analysis of observational studies. PLoS Med. 2011, 8, e1001048. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.O.; Mann, B.; Gao, G.; Hankins, J.S.; Humann, J.; Giardina, J.; Faverio, P.; Restrepo, M.I.; Halade, G.V.; Mortensen, E.M.; et al. Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog. 2014, 10, e1004383. [Google Scholar] [CrossRef]
- Gilley, R.P.; Gonzalez-Juarbe, N.; Shenoy, A.T.; Reyes, L.F.; Dube, P.H.; Restrepo, M.I.; Orihuela, C.J. Infiltrated Macrophages Die of Pneumolysin-Mediated Necroptosis following Pneumococcal Myocardial Invasion. Infect. Immun. 2016, 84, 1457–1469. [Google Scholar] [CrossRef] [Green Version]
- Alhamdi, Y.; Neill, D.R.; Abrams, S.T.; Malak, H.A.; Yahya, R.; Barrett-Jolley, R.; Wang, G.; Kadioglu, A.; Toh, C.H. Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection. PLoS Pathog. 2015, 11, e1004836. [Google Scholar] [CrossRef] [Green Version]
- Reyes, L.F.; Restrepo, M.I.; Hinojosa, C.A.; Soni, N.J.; Anzueto, A.; Babu, B.L.; Gonzalez-Juarbe, N.; Rodriguez, A.H.; Jimenez, A.; Chalmers, J.D.; et al. Severe Pneumococcal Pneumonia Causes Acute Cardiac Toxicity and Subsequent Cardiac Remodeling. Am. J. Respir. Crit. Care Med. 2017, 196, 609–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazaz, R.F.S.; Dockrell, D. Increased atherosclerotic plaque macrophage content following Streptococcus pneumonia. BMJ 2015. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Reyes, L.F.; Anzueto, A. Complication of Community-Acquired Pneumonia (Including Cardiac Complications). Semin. Respir. Crit. Care Med. 2016, 37, 897–904. [Google Scholar] [PubMed]
- Nel, J.G.; Durandt, C.; Mitchell, T.J.; Feldman, C.; Anderson, R.; Tintinger, G.R. Pneumolysin Mediates Platelet Activation In Vitro. Lung 2016, 194, 589–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.C.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015, 313, 264–274. [Google Scholar] [CrossRef]
- Isturiz, R.E.; Hall-Murray, C.; McLaughlin, J.M.; Snow, V.; Schmoele-Thoma, B.; Webber, C.; Thompson, A.; Scott, D.A. Pneumococcal conjugate vaccine use for the prevention of pneumococcal disease in adults < 50 years of age. Expert Rev. Vaccines 2018, 17, 45–55. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Vaccination. Available online: https://www.cdc.gov/vaccines/adults/rec-vac/heart-disease-home.html (accessed on 1 April 2022).
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation 2013, 128, e240–e327. [Google Scholar]
- Tsivgoulis, G.; Katsanos, A.H.; Zand, R.; Ishfaq, M.F.; Malik, M.T.; Karapanayiotides, T.; Voumvourakis, K.; Tsiodras, S.; Parissis, J. The association of adult vaccination with the risk of cerebrovascular ischemia: A systematic review and meta-analysis. J. Neurol. Sci. 2018, 386, 12–18. [Google Scholar] [CrossRef]
- Fountoulaki, K.; Tsiodras, S.; Polyzogopoulou, E.; Olympios, C.; Parissis, J. Beneficial Effects of Vaccination on Cardiovascular Events: Myocardial Infarction, Stroke, Heart Failure. Cardiology 2018, 141, 98–106. [Google Scholar] [CrossRef]
- Ren, S.; Newby, D.; Li, S.C.; Walkom, E.; Miller, P.; Hure, A.; Attia, J. Effect of the adult pneumococcal polysaccharide vaccine on cardiovascular disease: A systematic review and meta-analysis. Open Heart 2015, 2, e000247. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Almas, T.; Peng Ang, S.; David, S.; Shama, N.; Storozhenko, T.; Lnu, K.; Parmar, G.; Qaiser, S.; Naz, S.; et al. Symptomatology, prognosis and clinical findings of STEMI as a ramification of COVID-19: A systematic review and proportion meta-analysis. Ann. Med. Surg. 2022, 76, 103429. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Nepal, G.; Dijamco, P.; Ishak, A.; Dagar, M.; Sarfraz, Z.; Shama, N.; Sarfraz, A.; Lnu, K.; Mitra, S.; et al. Cerebral Venous Sinus Thrombosis Following COVID-19 Vaccination: A Systematic Review. J. Prim. Care Community Health 2022, 13, 21501319221074450. [Google Scholar] [CrossRef] [PubMed]
- The EndNote Team. EndNote, EndNote 20; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Patsopoulos, N.A.; Evangelou, E.; Ioannidis, J.P. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008, 37, 1148–1157. [Google Scholar] [CrossRef] [Green Version]
- StataCorp Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021.
- Gilbertson, D.T.; Guo, H.; Arneson, T.J.; Collins, A.J. The association of pneumococcal vaccination with hospitalization and mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 2934–2939. [Google Scholar] [CrossRef]
- Hung, I.F.N.; Leung, A.Y.M.; Chu, D.W.S.; Leung, D.; Cheung, T.; Chan, C.-K.; Lam, C.L.K.; Liu, S.-H.; Chu, C.-M.; Ho, P.-L.; et al. Prevention of Acute Myocardial Infarction and Stroke among Elderly Persons by Dual Pneumococcal and Influenza Vaccination: A Prospective Cohort Study. Clin. Infect. Dis. 2010, 51, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Tseng, H.F.; Slezak, J.M.; Quinn, V.P.; Sy, L.S.; Van den Eeden, S.K.; Jacobsen, S.J. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA 2010, 303, 1699–1706. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Gondar, O.; Vila-Corcoles, A.; Rodriguez-Blanco, T.; de Diego-Cabanes, C.; Hospital-Guardiola, I.; Jariod-Pamies, M. Evaluating the clinical effectiveness of pneumococcal vaccination in preventing myocardial infarction: The CAPAMIS study, three-year follow-up. Vaccine 2014, 32, 252–257. [Google Scholar] [CrossRef]
- Zahid, M.; Singla, I.; Good, C.; Stone, R.; Kim, S.; Fine, M.; Sonel, A. Associations between Pneumococcal Vaccinationand Adverse Outcomes in Patients with Suspected Acute Coronary Syndrome. Adv. Infect. Dis. 2012, 2, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Eurich, D.T.; Johnstone, J.J.; Minhas-Sandhu, J.K.; Marrie, T.J.; Majumdar, S.R. Pneumococcal vaccination and risk of acute coronary syndromes in patients with pneumonia: Population-based cohort study. Heart 2012, 98, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Patel, K.; Fonarow, G.C.; Morgan, C.J.; Butler, J.; Bittner, V.; Kulczycki, A.; Kheirbek, R.E.; Aronow, W.S.; Fletcher, R.D.; et al. Higher risk for incident heart failure and cardiovascular mortality among community-dwelling octogenarians without pneumococcal vaccination. ESC Heart Fail 2016, 3, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Jiang, L.; Friedmann, P.D.; Trivedi, A. Association between process quality measures for heart failure and mortality among US veterans. Am. Heart J. 2014, 168, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila-Corcoles, A.; Ochoa-Gondar, O.; Rodriguez-Blanco, T.; de Diego-Cabanes, C.; Satue-Gracia, E.; Vila-Rovira, A.; Torrente Fraga, C. Evaluating clinical effectiveness of pneumococcal vaccination in preventing stroke: The CAPAMIS Study, 3-year follow-up. J. Stroke Cerebrovasc. Dis. 2014, 23, 1577–1584. [Google Scholar] [CrossRef]
- Jackson, L.A.; Yu, O.; Heckbert, S.R.; Psaty, B.M.; Malais, D.; Barlow, W.E.; Thompson, W.W.; Vaccine Safety Datalink Study Group. Influenza Vaccination Is Not Associated with a Reduction in the Risk of Recurrent Coronary Events. Am. J. Epidemiol. 2002, 156, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Bond, T.C.; Spaulding, A.C.; Krisher, J.; McClellan, W. Mortality of dialysis patients according to influenza and pneumococcal vaccination status. Am. J. Kidney Dis. 2012, 60, 959–965. [Google Scholar] [CrossRef]
- Ihara, H.; Kikuchi, K.; Taniguchi, H.; Fujita, S.; Tsuruta, Y.; Kato, M.; Mitsuishi, Y.; Tajima, K.; Kodama, Y.; Takahashi, F.; et al. 23-valent pneumococcal polysaccharide vaccine improves survival in dialysis patients by preventing cardiac events. Vaccine 2019, 37, 6447–6453. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Yu, C.-C.; Hu, H.-C.; Thai, Y.-H. No preventive effects of 23-valent pneumococcal polysaccharide vaccine in patients with chronic renal failure. Eur. Respir. J. 2016, 48, PA612. [Google Scholar]
- Siriwardena, A.N.; Asghar, Z.; Coupland, C.C. Influenza and pneumococcal vaccination and risk of stroke or transient ischaemic attack-matched case control study. Vaccine 2014, 32, 1354–1361. [Google Scholar] [CrossRef]
- Lamontagne, F.; Garant, M.P.; Carvalho, J.C.; Lanthier, L.; Smieja, M.; Pilon, D. Pneumococcal vaccination and risk of myocardial infarction. CMAJ 2008, 179, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques Antunes, M.; Duarte, G.S.; Brito, D.; Borges, M.; Costa, J.; Ferreira, J.J.; Pinto, F.J.; Caldeira, D. Pneumococcal vaccination in adults at very high risk or with established cardiovascular disease: Systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Zhang, A.; Gillman, E.; Bessai, K.; Parhar, K.; Vadlamudi, N.K. The protective effect of pneumococcal vaccination on cardiovascular disease in adults: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 99, 204–213. [Google Scholar] [CrossRef]
- Vlachopoulos, C.V.; Terentes-Printzios, D.G.; Aznaouridis, K.A.; Pietri, P.G.; Stefanadis, C.I. Association between pneumococcal vaccination and cardiovascular outcomes: A systematic review and meta-analysis of cohort studies. Eur. J. Prev. Cardiol. 2015, 22, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Ciszewski, A. Cardioprotective effect of influenza and pneumococcal vaccination in patients with cardiovascular diseases. Vaccine 2018, 36, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Binder, C.J.; Horkko, S.; Dewan, A.; Chang, M.K.; Kieu, E.P.; Goodyear, C.S.; Shaw, P.X.; Palinski, W.; Witztum, J.L.; Silverman, G.J. Pneumococcal vaccination decreases atherosclerotic lesion formation: Molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003, 9, 736–743. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Madjid, M.; Musher, D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010, 10, 83–92. [Google Scholar] [CrossRef]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Rombauts, A.; Abelenda-Alonso, G.; Càmara, J.; Lorenzo-Esteller, L.; González-Díaz, A.; Sastre-Escolà, E.; Gudiol, C.; Dorca, J.; Tebé, C.; Pallarès, N.; et al. Host- and Pathogen-Related Factors for Acute Cardiac Events in Pneumococcal Pneumonia. Open Forum Infect. Dis. 2020, 7, ofaa522. [Google Scholar] [CrossRef]
- Horkko, S.; Bird, D.A.; Miller, E.; Itabe, H.; Leitinger, N.; Subbanagounder, G.; Berliner, J.A.; Friedman, P.; Dennis, E.A.; Curtiss, L.K.; et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Investig. 1999, 103, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, P.X.; Horkko, S.; Chang, M.K.; Curtiss, L.K.; Palinski, W.; Silverman, G.J.; Witztum, J.L. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Investig. 2000, 105, 1731–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.; Hure, A.; Peel, R.; D’Este, C.; Abhayaratna, W.; Tonkin, A.; Hopper, I.; Thrift, A.G.; Levi, C.; Sturm, J.; et al. Rationale and design of a randomized controlled trial of pneumococcal polysaccharide vaccine for prevention of cardiovascular events: The Australian Study for the Prevention through Immunization of Cardiovascular Events (AUSPICE). Am. Heart J. 2016, 177, 58–65. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Study Design | Country | Study Population | Follow Up, Years | No of Patients, n (PV/Control) | Outcomes | Outcome Adjustment |
|---|---|---|---|---|---|---|---|---|
| Gilbertson et al. [31] | 2011 | prospective | USA | Hemodialysis patients | 0.5 | 25,091/93,442 | All-cause mortality, Cardiovascular mortality | Patient demographics, comorbidity, and influenza vaccinations |
| Hung et al. [32] | 2010 | prospective | Hong Kong | Patients aged ≥65 years and had ≥1 of the following chronic illness: asthma, chronic obstructive pulmonary disease (COPD), coronary artery disease, hypertension, diabetes mellitus, stroke, chronic renal or liver disease, or malignancy. | 1.25 | 1875/25,393 | All-cause mortality, Stroke, Acute MI | Sex and COPD |
| Tseng et al. [33] | 2010 | prospective | USA | Men aged 45 to 69 | 4.7 | 36,309/47,861 | Incidence of Stroke and Acute MI | Age, region, race/ethnicity, smoking, BMI, physical inactivity, income, education, history of MI, history of stroke, history of PAD, high cholesterol, high BP, DM, other HD, nutrition, alcohol consumption, outpatient visits, sedentary status, influenza vaccinations |
| Ochoa-Gondar et al. [34] | 2014 | prospective | Spain | Adults aged 60 years old and older, with or without prior h/o CAD | 3 | 8981/18,223 | All-cause mortality, death from MI | Age, sex, influenza vaccination status, number of outpatient visits in previous 12-month, nursing-home residence, history of pneumonia, cerebrovascular disease, chronic pulmonary disease, chronic heart disease, chronic liver disease, chronic nephropathy, DM, cancer, dementia, immunodeficiency, HTN, hypercholesterolemia, obesity, alcoholism, smoking, and immunosuppressive medication |
| Zahid et al. [35] | 2012 | retrospective | USA | Patients with suspected ACS | 0.5 | 507/579 | All-cause mortality, acute MI | Propensity score for pneumococcal vaccination. Adjusted for variables including influenza vaccination only, pneumococcal and influenza vaccinations, age (per year), SBP < 90 mmHg, pulmonary edema on admission, hemoglobin < 11.5 gm/dL, left ventricular ejection fraction < 35%, smoking (past/current), increased troponin, DM, statins, and missing data |
| Eurich et al. [36] | 2012 | prospective | Canada | Patients aged >17 years with pneumonia | 0.25 | 725/5446 | Fatal and non-fatal ACS | Pneumonia severity based on the PSI; comorbidities including COPD, DM, CAD, functional status, smoking status and CV and other medications |
| Ahmed et al. [37] | 2015 | prospective | USA | Community dwelling adults aged 65 and above, with h/o MI or coronary heart disease | 13 | 1424/3866 | All-cause mortality, cardiovascular mortality | Age ≥ 85 years, sex, race, married, education college or higher, income ≥ USD 25 K, smoking ≥32 pack years, walk blocks last week ≥ 10, body mass index ≥ 25 kg/m2, instrumental activities of daily living ≥1, Centers for Epidemiologic Studies Depression (CES-D) scale score, MMSE, influenza vaccination, CHD, HTN, DM, stroke, acute MI, AF, LVH, LV systolic dysfunction, LBBB, CKD, COPD, pneumonia, serum CRP ≥ 2.4 mg/L |
| Wu et al. [38] | 2014 | retrospective | USA | Adults with HF | 1 | 7108/586 | All-cause mortality | Age; sex; race; hospital days last 6 months; number of hospitalizations prior 6 months; prior HF hospitalization; Elixhauser risk index, prior MI; fiscal year of the assessment; hematocrit, MABP, pulse, creatinine clearance, and clustering within hospitals |
| Vila-Corcoles et al. [39] | 2014 | prospective | Spain | Adults aged 60 years old and older, with or without prior CVA | 3 | 8981/1823 | All-cause mortality | Age, sex, influenza vaccination status, number of outpatient visits in previous 12 months, nursing-home residence, history of pneumonia, coronary artery disease, cerebrovascular disease, chronic pulmonary disease, chronic heart disease, chronic liver disease, chronic nephropathy, DM, cancer, immunodeficiency, dementia, HTN, hypercholesterolemia, obesity, alcoholism, smoking, and immunosuppressive medication |
| Jackson et al. [40] | 2002 | retrospective | USA | Patients with a first nonfatal myocardial infarction | 2.3 | 661/1378 | Acute MI | Age, sex, shock, or severe CHF (defined as requiring hemodynamic monitoring and/or vasopressor support) during hospitalization for the incident myocardial infarction, smoking status, DM, HTN, chronic CHF, COPD/asthma, and cardiac medication use |
| Bond et al. [41] | 2012 | retrospective | USA | Dialysis patients | 1 | 1297/20,180 | All-cause mortality | Age, race, sex, time on dialysis (vintage), modality (hemodialysis, continuous cyclic peritoneal dialysis, or continuous ambulatory peritoneal dialysis), diabetes as primary cause of ESRD (yes or no), comorbid conditions at dialysis therapy initiation (congestive heart failure, cerebrovascular disease, peripheral vascular disease, history of hypertension, chronic obstructive pulmonary disease, and malignant neoplasm), and mean monthly patient laboratory values for albumin, hemoglobin, and Kt/V during the 3-month influenza vaccination period |
| Ihara et al. [42] | 2019 | retrospective | Japan | Dialysis patients | 5 | 255/255 | All-cause mortality, CV mortality | Propensity score-matched using variables including age, sex, body mass index (BMI), duration of dialysis, serum level of albumin, influenza vaccination in 2010, history of arteriosclerotic heart disease, chronic heart failure, peripheral vascular disease, and diabetes mellitus (DM) |
| Hsieh et al. [43] | 2016 | retrospective | Taiwan | Patients aged >50 years with chronic renal failure on maintenance hemodialysis | 5 | 168/377 | All-cause mortality, acute MI, stroke | - |
| Siriwardena et al. [44] | 2014 | case–control | UK | Adults (>40) with a first diagnosis of MI | 1 | 26,847/13,615 | Stroke | Asthma, COPD, or CAD, stroke or TIA, DM, hyperlipidemia, splenectomy, chronic liver disease, CRF, immunosuppression, HIV/AIDS, family history of AMI, PVD, HTN, smoking status, treatment with acetylsalicylic acid or statins, or antihypertensives, GP consultations, BMI |
| Lamontagne et al. [45] | 2008 | case–control | Canada | Patients at risk of MI | 1.8 | 536/4459 | Acute MI | Matched for age, sex and hospitalization index date. Comparisons were adjusted for COPD, CRF, DM, previous S. pneumoniae infection, splenectomy |
| Variables | Gilbertson 2011 [31] | Hung 2010 [32] | Tseng 2010 [33] | Ochoa-Gondar 2014 [34] | Zahid 2012 [35] | Eurich 2012 [36] | Ahmed 2015 [37] | Wu 2014 [38] | Vila-Corcoles 2014 [39] | Jackson 2002 [40] | Bond 2012 [41] | Ihara 2019 [42] | Hsieh 2016 [43] | Siriwardena 2014 [44] | Lamontagne 2008 [45] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (n) PV/Placebo | 25,091/93,442 | 1875/25,393 | 36,309/47,861 | 8981/18,223 | 507/579 | 725/5446 | 1424/3866 | 7108/586 | 8981/18,223 | 661/1378 | 1297/20,180 | 255/255 | 168/377 | 26,847/13,615 | 536/4459 |
| Age, years (Mean) | /55.2 | - | 68.9/ | 71.82/64.55 | - | - | 59.3/59.8 | 61.3/62 | - | - | - | ||||
| Male, % | 52.3/53.2 | 45/47 | - | 45.2/44 | 97.8/97.4 | 48/53 | 41.57/42.03 | 98.2/98.8 | 45.2/44.3 | - | 48.7/52.0 | 68.2/67.5 | - | - | - |
| Obesity | - | - | 27.99/24.68 | 37.3/17.3 | - | - | - | - | 35.1/27.9 | - | - | - | - | 38.3/54.5 | - |
| Past Influenza Vaccination, % | 89.6/71 | - | 82.1/38.4 | 69/100 | 90/3 | 77.67/31.14 | - | 82.1/38.4 | - | -/70.3 | 78.4/74.9 | - | 0/0 | - | |
| Comorbidities | |||||||||||||||
| HTN, % | 29/30.3 | 59.8/60.7 | 45.23/30.3 | 59.1/50.7 | 73.4/65.5 | - | 60.11/57.55 | - | 59.1/50.7 | - | 78.9/80.1 | - | - | 50.3/26.2 | - |
| HLD, % | - | - | 46.53/35.8 | 40.2/34.9 | - | - | - | - | 40.2/34.9 | - | - | - | - | 13.4/6.6 | - |
| DM, % | 62.1/59.4 | 24.5/24.1 | 20.57/6.2 | 24.4/20.4 | 41.8/36.96 | 90/3 | 15.03/15.7 | - | 24.4/20.4 | - | 24.1/22.8 | 60/61.2 | - | 16/4.2 | - |
| Smoker, % | - | 14.8/13.5 | 62.78/53.31 | 35.1/29.5 | 67.7/72.9 | 52.69/33.29 | - | - | 35.1/29.46 | - | 7.3/5.9 | - | - | 62.3/38.9 | - |
| COPD, % | 26/24.1 | 3.9/2 | - | 8.26/7.8 | - | 39/15 | 19.6/9.7 | - | 8.26/7.8 | - | 4.3/3.9 | - | - | 23.4/7.8 | - |
| CHF, % | 51.1/49.8 | 8.7/7.9 | 3.9/1.6 | 14.7/11.4 | 18/15.9 | - | 7.4/7.2 | 24.6/20.3 | - | 22.4/22.0 | 23.9/26.7 | - | - | - | |
| Chronic Liver Disease, % | 12.4/13.3 | 0.3/0.3 | - | 2.66/2.1 | - | - | - | - | 2.66/2.1 | - | - | 2/5.5 | - | 0.5/0.2 | - |
| Previous MI, % | - | 1/1.2 | - | - | 24.9/21.76 | - | 8.15/7.79 | 34.8/24.4 | - | - | - | - | - | - | - |
| Previous Stroke, % | 24.3/23 | 0.39/7.1 | - | 5.4/4.43 | 14.8/9.67 | - | 4.78/3.52 | - | 5.4/4.43 | - | - | 20.4/23.5 | - | - | - |
| Kidney Disease, % | 100/100 | 2.6/2.3 | - | 2.38/2.44 | 16.8/16.75 | 13/7 | - | - | 2.38/2.4 | - | 100/100 | 100/100 | 100/100 | 12.7/2.7 | - |
| Meta-Regression Variables | All-Cause Mortality | MI | ||
|---|---|---|---|---|
| Coefficient | p * | Coefficient | p * | |
| Demographics | ||||
| Age | 0.017 | 0.267 | 0.009 | 0.814 |
| Male | −0.011 | 0.027 | −0.009 | 0.466 |
| Comorbidities | ||||
| HTN | −0.002 | 0.727 | - | - |
| DM | −0.004 | 0.432 | 0.005 | 0.758 |
| HLD | - | - | - | - |
| Prior CVA | −0.008 | 0.589 | - | - |
| Prior MI | - | - | - | - |
| HF | 0.002 | 0.765 | ||
| CVD | −0.011 | 0.161 | - | - |
| COPD | 0.007 | 0.490 | - | - |
| CKD | 0.001 | 0.593 | −0.001 | 0.806 |
| Smoking | - | - | −0.002 | 0.876 |
| Prior Influenza vaccine | 0.011 | 0.198 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, V.; Ang, S.P.; Lnu, K.; Ishak, A.; Pokhrel, N.B.; Chia, J.E.; Hajra, A.; Biswas, M.; Matetic, A.; Dhatt, R.; et al. Effect of Pneumococcal Vaccine on Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3799. https://doi.org/10.3390/jcm11133799
Jaiswal V, Ang SP, Lnu K, Ishak A, Pokhrel NB, Chia JE, Hajra A, Biswas M, Matetic A, Dhatt R, et al. Effect of Pneumococcal Vaccine on Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(13):3799. https://doi.org/10.3390/jcm11133799
Chicago/Turabian StyleJaiswal, Vikash, Song Peng Ang, Kriti Lnu, Angela Ishak, Nishan Babu Pokhrel, Jia Ee Chia, Adrija Hajra, Monodeep Biswas, Andrija Matetic, Ravinder Dhatt, and et al. 2022. "Effect of Pneumococcal Vaccine on Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 13: 3799. https://doi.org/10.3390/jcm11133799
APA StyleJaiswal, V., Ang, S. P., Lnu, K., Ishak, A., Pokhrel, N. B., Chia, J. E., Hajra, A., Biswas, M., Matetic, A., Dhatt, R., & Mamas, M. A. (2022). Effect of Pneumococcal Vaccine on Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(13), 3799. https://doi.org/10.3390/jcm11133799