An Ultra-Short Measure of Excessive Daytime Sleepiness Is Related to Circadian Biological Rhythms: The French Psychometric Validation of the Barcelona Sleepiness Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Research Tools
2.3. Statistical Analysis
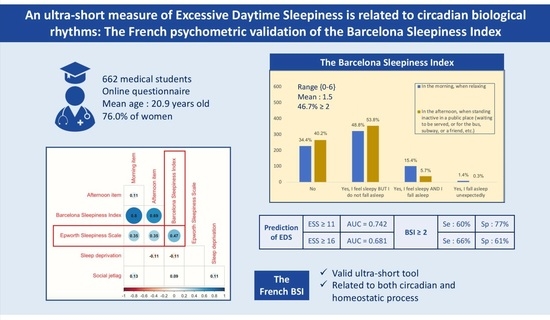
3. Results
3.1. Sample Description
3.2. Ability of BSI Score to Predict EDS
3.3. Concurrent and External Validity of the BSI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, T.B. Epidemiology of Daytime Sleepiness: Definitions, Symptomatology, and Prevalence. J. Clin. Psychiatry 2004, 65 (Suppl. S16), 12–16. [Google Scholar] [PubMed]
- Léger, D.; Stepnowsky, C. The Economic and Societal Burden of Excessive Daytime Sleepiness in Patients with Obstructive Sleep Apnea. Sleep Med. Rev. 2020, 51, 101275. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J. Sleep Health: Can We Define It? Does It Matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, N. Objective and Subjective Measurement of Excessive Sleepiness. Sleep Med. Clin. 2012, 7, 219–232. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaida, K.; Takahashi, M.; Akerstedt, T.; Nakata, A.; Otsuka, Y.; Haratani, T.; Fukasawa, K. Validation of the Karolinska Sleepiness Scale against Performance and EEG Variables. Clin. Neurophysiol. 2006, 117, 1574–1581. [Google Scholar] [CrossRef]
- MacLean, A.W.; Fekken, G.C.; Saskin, P.; Knowles, J.B. Psychometric Evaluation of the Stanford Sleepiness Scale. J. Sleep Res. 1992, 1, 35–39. [Google Scholar] [CrossRef]
- Kaminska, M.; Jobin, V.; Mayer, P.; Amyot, R.; Perraton-Brillon, M.; Bellemare, F. The Epworth Sleepiness Scale: Self-Administration versus Administration by the Physician, and Validation of a French Version. Can. Respir. J. 2010, 17, 438676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendzerska, T.B.; Smith, P.M.; Brignardello-Petersen, R.; Leung, R.S.; Tomlinson, G.A. Evaluation of the Measurement Properties of the Epworth Sleepiness Scale: A Systematic Review. Sleep Med. Rev. 2014, 18, 321–331. [Google Scholar] [CrossRef]
- Miletin, M.S.; Hanly, P.J. Measurement Properties of the Epworth Sleepiness Scale. Sleep Med. 2003, 4, 195–199. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Hallvig, D.; Kecklund, G. Normative Data on the Diurnal Pattern of the Karolinska Sleepiness Scale Ratings and Its Relation to Age, Sex, Work, Stress, Sleep Quality and Sickness Absence/Illness in a Large Sample of Daytime Workers. J. Sleep Res. 2017, 26, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza-Vargas, S.; Giannouli, E.; Younes, M. Enhancements to the Multiple Sleep Latency Test. Nat. Sci. Sleep 2016, 8, 145–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, D.C.; Taylor, D.J.; Okonkwo, R.; Becker, P.M.; Jamieson, A.O.; Schmidt-Nowara, W.; Rosenthal, L.D. The Time of Day Sleepiness Scale to Assess Differential Levels of Sleepiness across the Day. J. Psychosom. Res. 2009, 67, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Seda, G.; Han, T.S. Effect of Obstructive Sleep Apnea on Neurocognitive Performance. Sleep Med. Clin. 2020, 15, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; DuMont, K. Using Focus Groups to Facilitate Culturally Anchored Research. Am. J. Community Psychol. 1993, 21, 775–806. [Google Scholar] [CrossRef]
- Guaita, M.; Salamero, M.; Vilaseca, I.; Iranzo, A.; Montserrat, J.M.; Gaig, C.; Embid, C.; Romero, M.; Serradell, M.; León, C.; et al. The Barcelona Sleepiness Index: A New Instrument to Assess Excessive Daytime Sleepiness in Sleep Disordered Breathing. J. Clin. Sleep Med. 2015, 11, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Dobrow, M.J.; Hagens, V.; Chafe, R.; Sullivan, T.; Rabeneck, L. Consolidated Principles for Screening Based on a Systematic Review and Consensus Process. CMAJ 2018, 190, E422–E429. [Google Scholar] [CrossRef] [Green Version]
- Jahrami, H.; Dewald-Kaufmann, J.; Faris, M.A.-I.; AlAnsari, A.M.S.; Taha, M.; AlAnsari, N. Prevalence of Sleep Problems among Medical Students: A Systematic Review and Meta-Analysis. J. Public Health 2020, 28, 605–622. [Google Scholar] [CrossRef]
- Shapiro, C.M.; Auch, C.; Reimer, M.; Kayumov, L.; Heslegrave, R.; Huterer, N.; Driver, H.; Devins, G.M. A New Approach to the Construct of Alertness. J. Psychosom. Res. 2006, 60, 595–603. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an Outcome Measure for Insomnia Research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.; Löwe, B. An Ultra-Brief Screening Scale for Anxiety and Depression: The PHQ-4. Psychosomatics 2009, 50, 613–621. [Google Scholar] [CrossRef]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Reed, D.L.; Sacco, W.P. Measuring Sleep Efficiency: What Should the Denominator Be? J. Clin. Sleep Med. 2016, 12, 263–266. [Google Scholar] [CrossRef]
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology 2019, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s Updated Sleep Duration Recommendations: Final Report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Beauvalet, J.C.; Quiles, C.L.; de Oliveira, M.A.B.; Ilgenfritz, C.A.V.; Hidalgo, M.P.L.; Tonon, A.C. Social Jetlag in Health and Behavioral Research: A Systematic Review. CPT 2017, 7, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Y.; Feng, Z.; Yi, X. A General Introduction to Adjustment for Multiple Comparisons. J. Thorac. Dis. 2017, 9, 1725–1729. [Google Scholar] [CrossRef] [Green Version]
- Shahid, A.; Chung, S.A.; Maresky, L.; Danish, A.; Bingeliene, A.; Shen, J.; Shapiro, C.M. The Toronto Hospital Alertness Test Scale: Relationship to Daytime Sleepiness, Fatigue, and Symptoms of Depression and Anxiety. Nat. Sci. Sleep 2016, 8, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.W.; Song, M.L.; Morin, C.M. Validation of a Korean Version of the Insomnia Severity Index. J. Clin. Neurol. 2014, 10, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Nuyen, B.A.; Fox, R.S.; Malcarne, V.L.; Wachsman, S.I.; Sadler, G.R. Excessive Daytime Sleepiness as an Indicator of Depression in Hispanic Americans. Hisp. Health Care Int. 2016, 14, 116–123. [Google Scholar] [CrossRef]
- Taillard, J.; Sagaspe, P.; Philip, P.; Bioulac, S. Sleep Timing, Chronotype and Social Jetlag: Impact on Cognitive Abilities and Psychiatric Disorders. Biochem. Pharmacol. 2021, 191, 114438. [Google Scholar] [CrossRef] [PubMed]
- Nowack, K.; Van Der Meer, E. The Synchrony Effect Revisited: Chronotype, Time of Day and Cognitive Performance in a Semantic Analogy Task. Chronobiol. Int. 2018, 35, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Arand, D.L. Arousal Components Which Differentiate the MWT from the MSLT. Sleep 2001, 24, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taillard, J.; Philip, P.; Coste, O.; Sagaspe, P.; Bioulac, B. The Circadian and Homeostatic Modulation of Sleep Pressure during Wakefulness Differs between Morning and Evening Chronotypes. J. Sleep Res. 2003, 12, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Andrillon, T.; Solelhac, G.; Bouchequet, P.; Romano, F.; Le Brun, M.-P.; Brigham, M.; Chennaoui, M.; Léger, D. Revisiting the Value of Polysomnographic Data in Insomnia: More than Meets the Eye. Sleep Med. 2020, 66, 184–200. [Google Scholar] [CrossRef]
- Lusic Kalcina, L.; Valic, M.; Pecotic, R.; Pavlinac Dodig, I.; Dogas, Z. Good and Poor Sleepers among OSA Patients: Sleep Quality and Overnight Polysomnography Findings. Neurol. Sci. 2017, 38, 1299–1306. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Azad, M.C.; Fraser, K.; Rumana, N.; Abdullah, A.F.; Shahana, N.; Hanly, P.J.; Turin, T.C. Sleep Disturbances among Medical Students: A Global Perspective. J. Clin. Sleep Med. 2015, 11, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Koopman, A.D.M.; Rauh, S.P.; van ‘t Riet, E.; Groeneveld, L.; van der Heijden, A.A.; Elders, P.J.; Dekker, J.M.; Nijpels, G.; Beulens, J.W.; Rutters, F. The Association between Social Jetlag, the Metabolic Syndrome, and Type 2 Diabetes Mellitus in the General Population: The New Hoorn Study. J. Biol. Rhythms 2017, 32, 359–368. [Google Scholar] [CrossRef]
- Institut National du Sommeil et de la Vigilance (National Institute of Sleep and Alertness). Bien Dormir Pour Mieux Faire Face (Sleep Well to Cope Better). 2020. Available online: https://institut-sommeil-vigilance.org/bien-dormir-pour-mieux-faire-face-enquete-insv-mgen-2021 (accessed on 16 May 2022).
- Brown, F.C.; Buboltz, W.C.; Soper, B. Relationship of Sleep Hygiene Awareness, Sleep Hygiene Practices, and Sleep Quality in University Students. Behav. Med. 2002, 28, 33–38. [Google Scholar] [CrossRef]
- Bioulac, S.; Micoulaud-Franchi, J.-A.; Arnaud, M.; Sagaspe, P.; Moore, N.; Salvo, F.; Philip, P. Risk of Motor Vehicle Accidents Related to Sleepiness at the Wheel: A Systematic Review and Meta-Analysis. Sleep 2017, 40, zsx134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adan, A. A Chronobiological Approach to Addiction. J. Subst. Use 2013, 18, 171–183. [Google Scholar] [CrossRef]
- Chaput, J.-P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saunders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep Timing, Sleep Consistency, and Health in Adults: A Systematic Review. Appl. Physiol. Nutr. Metab. 2020, 45, S232–S247. [Google Scholar] [CrossRef] [PubMed]

| These questions refer to the sleepiness experienced during recent weeks | No | Yes, I feel sleepy BUT I do not fall asleep | Yes, I feel sleepy AND I fall asleep | Yes, I fall asleep unexpectedly | |
| Ces questions font référence à la somnolence ressentie dans les semaines précédentes | Non | Oui, Je me sens somnolent·e MAIS je ne m’endors Pas | Oui, je me sens Somnolent·e ET je m’endors | Oui, je m’endors subitement | |
| In the morning, when relaxing | Le matin, en se reposant | 228 (34.4%) | 323 (48.8%) | 102 (15.4%) | 9 (1.4%) |
| In the afternoon, when standing, inactive in a public place (waiting to be served, or for the bus, subway, or a friend, etc.) | Dans l’après-midi, en se tenant debout, inactif·ve dans un lieu public (en faisant la queue, ou en attendant le bus, le métro, ou un ami, etc.) | 266 (40.2%) | 356 (53.8%) | 38 (5.7%) | 2 (0.3%) |
| Total n = 662 | Mean ± Standard Deviation (Min:Max) | |
|---|---|---|
| Age (years) | 20.9 ± 2.6 (16:35) | |
| Sex | ||
| 159 (24.0%) | |
| 503 (76.0%) | |
| Year of study | ||
| 274 (41.4%) | |
| 388 (58.6%) | |
| Bedtime on workdays (hh:mm) | 23:30 ± 51 min | |
| Rise time on workdays (hh:mm) | 07:34 ± 45 min | |
| Sleep duration on workdays (hh:mm) | 7 h 25 ± 54 min | |
| 482 (72.8%) | |
| 144 (21.8%) | |
| 36 (5.4%) | |
| Bedtime on free days (hh:mm) | 23:57 ± 68 min | |
| Rise time on free days (hh:mm) | 09:07 ± 76 min | |
| Sleep duration on free days (hh:mm) | 8 h 27 ± 100 min | |
| Mean sleep duration (hh:mm) | 7 h 43 ± 53 min | |
| Mean sleep efficiency (mean percentage) | 92% ± 3% (73%:97%) | |
| 17 (2.6%) | |
| 566 (85.5%) | |
| 79 (11.9%) | |
| Social jetlag (hh:mm) | ||
| 68 (10.3%) | 55 ± 47 min (−85:225) |
| Insomnia Severity Scale (ISI) | ||
| 146 (22.0%) | 10.1 ± 5.3 (0:28) |
| Epworth Sleepiness Scale (ESS) | 12.5 ± 5.2 (0:24) | |
| 423 (63.9%) | |
| 186 (28.1%) | |
| Anxiety symptoms (PHQ-4) | 2.5 ± 1.9 (0:6) | |
| 277 (41.8%) | |
| Depressive symptoms | 2.0 ± 1.7 (0:6) | |
| 211 (31.9%) | |
| Barcelona Sleepiness Index (BSI) | 1.5 ± 1.0 (0:6) | |
| 309 (46.7%) |
| BSI Score | Prevalence | Sensitivity | Specificity | PPV | NPV | YI | |
|---|---|---|---|---|---|---|---|
| ESS score ≥ 11 | ≥1 | 85% | 94% | 29% | 70% | 72% | 0.23 |
| ≥2 | 47% | 60% | 77% | 82% | 52% | 0.37 | |
| ≥3 | 14% | 22% | 99% | 98% | 42% | 0.21 | |
| ESS score ≥ 16 | ≥1 | 85% | 96% | 19% | 32% | 92% | 0.15 |
| ≥2 | 47% | 66% | 61% | 40% | 82% | 0.27 | |
| ≥3 | 14% | 28% | 91% | 56% | 77% | 0.19 |
| BSI Score | ESS Score | THAT Score | ISI Score | Anxiety Symptoms | Depressive Symptoms | Mean Sleep Duration | Mean Sleep Efficiency | Social Jetlag | |
|---|---|---|---|---|---|---|---|---|---|
| BSI Morning item | 0.80 * | 0.35 * | −0.31 * | 0.30 * | 0.10 # | 0.16 * | −0.07 | −0.07 | 0.13 * |
| BSI Afternoon item | 0.69 * | 0.35 * | −0.29 * | 0.22 * | 0.13 * | 0.20 * | −0.11 # | −0.09 † | −0.02 |
| BSI score | - | 0.47 * | −0.40 * | 0.36 * | 0.15 * | 0.24 * | −0.11 # | −0.10 # | 0.09 † |
| ESS score | - | - | −0.34 * | 0.27 * | 0.17 * | 0.21 * | −0.08 | −0.05 | 0.01 |
| THAT score | - | - | - | −0.56 * | −0.46 * | −0.53 * | 0.17 * | 0.22 * | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, J.; Lopez, R.; Taillard, J.; D’Incau, E.; Fond, G.; Philip, P.; Micoulaud-Franchi, J.-A. An Ultra-Short Measure of Excessive Daytime Sleepiness Is Related to Circadian Biological Rhythms: The French Psychometric Validation of the Barcelona Sleepiness Index. J. Clin. Med. 2022, 11, 3892. https://doi.org/10.3390/jcm11133892
Coelho J, Lopez R, Taillard J, D’Incau E, Fond G, Philip P, Micoulaud-Franchi J-A. An Ultra-Short Measure of Excessive Daytime Sleepiness Is Related to Circadian Biological Rhythms: The French Psychometric Validation of the Barcelona Sleepiness Index. Journal of Clinical Medicine. 2022; 11(13):3892. https://doi.org/10.3390/jcm11133892
Chicago/Turabian StyleCoelho, Julien, Régis Lopez, Jacques Taillard, Emmanuel D’Incau, Guillaume Fond, Pierre Philip, and Jean-Arthur Micoulaud-Franchi. 2022. "An Ultra-Short Measure of Excessive Daytime Sleepiness Is Related to Circadian Biological Rhythms: The French Psychometric Validation of the Barcelona Sleepiness Index" Journal of Clinical Medicine 11, no. 13: 3892. https://doi.org/10.3390/jcm11133892
APA StyleCoelho, J., Lopez, R., Taillard, J., D’Incau, E., Fond, G., Philip, P., & Micoulaud-Franchi, J. -A. (2022). An Ultra-Short Measure of Excessive Daytime Sleepiness Is Related to Circadian Biological Rhythms: The French Psychometric Validation of the Barcelona Sleepiness Index. Journal of Clinical Medicine, 11(13), 3892. https://doi.org/10.3390/jcm11133892