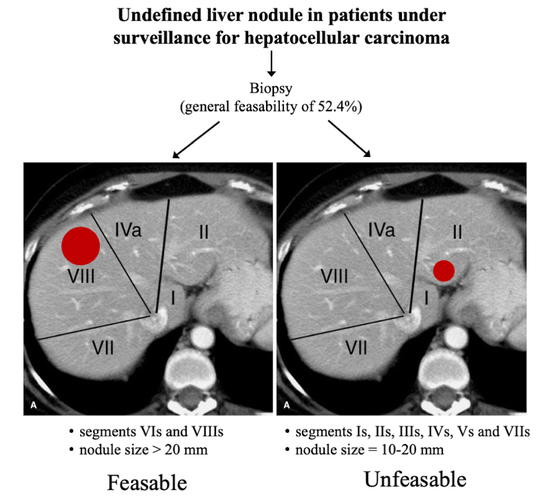
The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics and Imaging Technique
2.2. Imaging Processing and Analysis
2.3. Statistical Analysis
3. Results
3.1. Atypical Liver Nodule Characteristics
3.2. Feasibility of Liver Biopsy
3.3. Intra- and Inter-Reader Agreements
3.4. Factors Associated with the Unfeasibility of Biopsy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef]
- Paradis, V.; Zalinski, S.; Chelbi, E.; Guedj, N.; Degos, F.; Vilgrain, V.; Bedossa, P.; Belghiti, J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology 2009, 49, 851–859. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Pillai, A.; Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- Kansagara, D.; Papak, J.; Pasha, A.S.; O’Neil, M.; Freeman, M.; Relevo, R.; Quinones, A.; Motu’Apuaka, M.; Jou, J.H. Screening for hepatocellular carcinoma in chronic liver disease: A systematic review. Ann. Intern. Med. 2014, 161, 261–269. [Google Scholar] [CrossRef]
- Llovet, J.M.; Fuster, J.; Bruix, J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology 1999, 30, 1434–1440. [Google Scholar] [CrossRef]
- Terzi, E.; Piscaglia, F.; Forlani, L.; Mosconi, C.; Renzulli, M.; Bolondi, L.; Golfieri, R. TACE performed in patients with a single nodule of hepatocellular carcinoma. BMC Cancer 2014, 14, 601. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Tovoli, F.; Ielasi, L.; Gardini, A.C.; Granito, A.; Foschi, F.G.; Rovesti, G.; Negrini, G.; Orsi, G.; Renzulli, M.; Piscaglia, F. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J. Hepatol. 2019, 71, 1175–1183. [Google Scholar] [CrossRef]
- Terzi, E.; Terenzi, L.; Venerandi, L.; Croci, L.; Renzulli, M.; Mosconi, C.; Allegretti, G.; Granito, A.; Golfieri, R.; Bolondi, L.; et al. The ART score is not effective to select patients for transarterial chemoembolization retreatment in an Italian series. Dig. Dis. 2014, 32, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Park, H.J.; Jang, H.Y.; Kim, S.Y.; Lee, S.J.; Won, H.J.; Byun, J.H.; Choi, S.H.; Lee, S.S.; An, J.; Lim, Y.-S. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J. Hepatol. 2020, 72, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.V.; Huo, Y.R.; Trieu, N.; Mitchelle, A.; George, J.; He, E.; Lee, A.U.; Chang, J.; Yang, J. Noncontrast MRI for Hepatocellular Carcinoma Detection: A Systematic Review and Meta-analysis—A Potential Surveillance Tool? Clin. Gastroenterol. Hepatol. 2022, 20, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Garzillo, G.; Ascanio, S.; Renzulli, M. Focal lesions in the cirrhotic liver: Their pivotal role in gadoxetic acid-enhanced MRI and recognition by the Western guidelines. Dig. Dis. 2014, 32, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, G.; Giampalma, E.; Domenichelli, S.; Renzulli, M.; Golfieri, R. Calculation of conversion factors for effective dose for various interventional radiology procedures. Med. Phys. 2012, 39, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, J.M.; Sirlin, C.B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part I. Development, growth, and spread: Key pathologic and imaging aspects. Radiology 2014, 272, 635–654. [Google Scholar] [CrossRef]
- Forner, A.; Vilana, R.; Ayuso, C.; Bianchi, L.; Solé, M.; Ayuso, J.R.; Boix, L.; Sala, M.; Varela, M.; Llovet, J.M.; et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinom. Hepatology 2008, 47, 97–104, Correction in Hepatology 2008, 47, 769. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.M.; Lee, J.S.; Lee, H.Y.; Park, B.H.; Kim, Y.H.; Han, J.K.; Choi, B.I. Hepatocellular carcinoma: Diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015, 275, 97–109. [Google Scholar] [CrossRef]
- Pomfret, E.A.; Washburn, K.; Wald, C.; Nalesnik, M.A.; Douglas, D.; Russo, M.; Roberts, J.; Reich, D.J.; Schwartz, M.E.; Mieles, L.; et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010, 16, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Golfieri, R.; Bologna Liver Oncology Group (BLOG). Proposal of a new diagnostic algorithm for hepatocellular carcinoma based on the Japanese guidelines but adapted to the Western world for patients under surveillance for chronic liver disease. J. Gastroenterol. Hepatol. 2016, 31, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Yasuda, S.; Shiota, S.; Sone, Y.; Maeda, A.; Kaneoka, Y.; Kumada, T.; Tanaka, J. Pretreatment non-hypervascular hypointense nodules on Gd-EOB-DTPA-enhanced MRI as a predictor of hepatocellular carcinoma development after sustained virologic response in HCV infection. Aliment Pharmacol. Ther. 2021, 53, 1309–1316. [Google Scholar] [CrossRef]
- Sangiovanni, A.; Alimenti, E.; Gattai, R.; Filomia, R.; Parente, E.; Valenti, L.; Marzi, L.; Pellegatta, G.; Borgia, G.; Gambato, M.; et al. Undefined/non-malignant hepatic nodules are associated with early occurrence of HCC in DAA-treated patients with HCV-related cirrhosis. J. Hepatol. 2020, 73, 593–602. [Google Scholar] [CrossRef]
- Francque, S.M.; De Pauw, F.F.; Van den Steen, G.H.; Van Marck, E.A.; Pelckmans, P.A.; Michielsen, P.P. Biopsy of focal liver lesions: Guidelines, comparison of techniques and cost-analysis. Acta Gastroenterol. Belg. 2003, 66, 160–165. [Google Scholar] [PubMed]
- Giorgio, A.; Tarantino, L.; De Stefano, G.; Francica, G.; Esposito, F.; Perrotta, A.; Aloisio, V.; Farella, N.; Mariniello, N.; Coppola, C.; et al. Complications after interventional sonography of focal liver lesions: A 22-year single-center experience. J. Ultrasound Med. 2003, 22, 193–205. [Google Scholar] [CrossRef]
- Tovoli, F.; Renzulli, M.; Negrini, G.; Brocchi, S.; Ferrarini, A.; Andreone, A.; Benevento, F.; Golfieri, R.; Morselli-Labate, A.M.; Mastroroberto, M.; et al. Inter-operator variability and source of errors in tumour response assessment for hepatocellular carcinoma treated with sorafenib. Eur. Radiol. 2018, 28, 3611–3620. [Google Scholar] [CrossRef]
- Russo, V.; Renzulli, M.; La Palombara, C.; Fattori, R. Congenital diseases of the thoracic aorta. Role of MRI and MRA. Eur Radiol. 2006, 16, 676–684. [Google Scholar] [CrossRef]
- Russo, V.; Renzulli, M.; Buttazzi, K.; Fattori, R. Acquired diseases of the thoracic aorta: Role of MRI and MRA. Eur. Radiol. 2006, 16, 852–865. [Google Scholar] [CrossRef]
- Renzulli, M.; Dajti, E.; Ierardi, A.M.; Brandi, N.; Berzigotti, A.; Milandri, M.; Rossini, B.; Clemente, A.; Ravaioli, F.; Marasco, G.; et al. Validation of a standardized CT protocol for the evaluation of varices and porto-systemic shunts in cirrhotic patients. Eur. J. Radiol. 2022, 147, 110010. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Canellas, R.; Rosenkrantz, A.B.; Taouli, B.; Sala, E.; Saini, S.; Pedrosa, I.; Wang, Z.J.; Sahani, D.V. Abbreviated MRI Protocols for the Abdomen. Radiographics 2019, 39, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Leoni, S.; Piscaglia, F.; Golfieri, R.; Camaggi, V.; Vidili, G.; Pini, P.; Bolondi, L. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am. J. Gastroenterol. 2010, 105, 599–609. [Google Scholar] [CrossRef]
- Cabibbo, G.; Petta, S.; Barbàra, M.; Missale, G.; Virdone, R.; Caturelli, E.; Piscaglia, F.; Morisco, F.; Colecchia, A.; Farinati, F.; et al. A meta-analysis of single HCV-untreated arm of studies evaluating outcomes after curative treatments of HCV-related hepatocellular carcinoma. Liver Int. 2017, 37, 1157–1166. [Google Scholar] [CrossRef]
- Petta, S.; Cabibbo, G.; Barbara, M.; Attardo, S.; Bucci, L.; Farinati, F.; Giannini, E.; Tovoli, F.; Ciccarese, F.; Rapaccini, G.L.; et al. Hepatocellular carcinoma recurrence in patients with curative resection or ablation: Impact of HCV eradication does not depend on the use of interferon. Aliment Pharmacol. Ther. 2017, 45, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Renzulli, M.; Lucidi, V.; Corcioni, B.; Trevisani, F.; Bolondi, L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤2 cm) HCC in cirrhosis. Eur. Radiol. 2011, 21, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Brandi, N.; Pecorelli, A.; Pastore, L.V.; Granito, A.; Martinese, G.; Tovoli, F.; Simonetti, M.; Dajti, E.; Colecchia, A.; et al. Segmental Distribution of Hepatocellular Carcinoma in Cirrhotic Livers. Diagnostics 2022, 12, 834. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Nodules (N° 128) | p Value |
|---|---|---|
| Dimension (mm) [mean ± SD] Subgroups | 16.3 ± 5.2 | |
| 10–20 (n; %) | 107 (83.6) | ref. |
| 21–30 (n; %) | 12 (9.3) | <0.001 |
| 31–40 (n; %) | 9 (7.0) | <0.001 |
| Liver segment | ||
| 1 (n; %) | 0 | <0.001 |
| 2 (n; %) | 16 (12.5) | ref. |
| 3 (n; %) | 16 (12.5) | 1.000 |
| 4 (n; %) | 13 (10.1) | 0.554 |
| 5 (n; %) | 19 (14.8) | 0.585 |
| 6 (n; %) | 14 (10.9) | 0.698 |
| 7 (n; %) | 18 (14.1) | 0.713 |
| 8 (n; %) | 32 (25.0) | 0.010 |
| Location | ||
| Anterior, superficial (n; %) | 67 (52.3) | ref. |
| Posterior, superficial (n; %) | 25 (19.5) | <0.001 |
| Centrohepatic (n; %) | 28 (21.9) | <0.001 |
| Deep behind a fictitious line of the portal axis (n; %) | 8 (6.2) | <0.001 |
| Contrast behaviour | ||
| Arterial hypervascularisation (n; %) | 49 (38.3) | ref. |
| No arterial hypervascularisation (n; %) | 79 (61.7) | <0.001 |
| Characteristics | First Reader (No Biopsy = 76) | Second Reader (No Biopsy = 68) | p Value |
|---|---|---|---|
| Dimension (mm) [mean ± SD] Subgroups | 15.2 ± 5.1 | 15.3 ± 5.2 | 0.779 |
| 10–20 mm (n; %) | 66 (86.8) | 61 (89.7) | 0.578 |
| 21–30 mm (n; %) | 7 (9.2) | 4 (5.9) | 0.414 |
| 31–40 mm (n; %) | 3 (3.9) | 3 (4.4) | 1 |
| Liver segment | |||
| 1 (n; %) | 0 | ||
| 2 (n; %) | 6 (7.9) | 9 (13.2) | 0.479 |
| 3 (n; %) | 5 (6.6) | 7 (10.3) | 0.716 |
| 4 (n; %) | 8 (10.5) | 5 (7.4) | 0.433 |
| 5 (n; %) | 11 (14.5) | 12 (17.6) | 1 |
| 6 (n; %) | 5 (6.6) | 3 (4.4) | 0.678 |
| 7 (n; %) | 14 (18.4) | 10 (14.7) | 0.289 |
| 8 (n; %) | 27 (35.5) | 22 (32.4) | 0.237 |
| Location | |||
| Anterior, superficial (n; %) | 44 (57.9) | 36 (52.9) | 0.217 |
| Posterior, superficial (n; %) | 14 (18.4) | 12 (17.6) | 0.777 |
| Centrohepatic (n; %) | 14 (18.4) | 16(23.5) | 0.789 |
| Deep behind a fictitious line of the portal axis (n; %) | 4 (5.3) | 4 (5.9) | 1 |
| Contrast behaviour | |||
| Arterial hypervascularisation (n; %) | 16 (21.0) | 18 (26.5) | 0.832 |
| No arterial hypervascularisation (n; %) | 36 (47.4) | 42 (61.8) | 0.426 |
| Inter-Reader Agreement (95% CI) | Intra-Reader Agreement (95% CI) | |
|---|---|---|
| The PDF document | 0.185 (0.021–0.348) | 0.648 (0.513–0.783) |
| Complete contrast-enhanced CT study | 0.424 (0.269–0.579) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | p-Value | HR | 95% CI | p Value |
| Dimension (mm) Subgroups | 1.040 | 0.989–1.094 | 0.125 | |||
| 10–20 (n; %) | 2.652 | 0.991–7.100 | 0.052 | 3.639 | 1.257–10.381 | 0.016 |
| 21–30 (n; %) | 0.406 | 0.116–1.425 | 0.159 | |||
| 31–40 (n; %) | 0.415 | 0.099–1.739 | 0.229 | |||
| Liver segment | ||||||
| 1 (n; %) | -- | |||||
| 2 (n; %) | 1.155 | 0.402–3.317 | 0.789 | |||
| 3 (n; %) | 0.650 | 0.226–1.868 | 0.424 | |||
| 4 (n; %) | 0.516 | 0.159–1.672 | 0.270 | |||
| 5 (n; %) | 1.622 | 0.594–4.432 | 0.345 | |||
| 6 (n; %) | 0.206 | 0.054–0.777 | 0.020 | 0.220 | 0.057–0.859 | 0.029 |
| 7 (n; %) | 1.121 | 0.411–3.053 | 0.824 | |||
| 8 (n; %) | 5.180 | 1.840–14.577 | 0.002 | 2.416 | 0.956–6.049 | 0.060 |
| Location | ||||||
| Anterior, superficial | 1.052 | 0.525–2.109 | 0.885 | |||
| Posterior, superficial | 0.775 | 0.323–1.859 | 0.568 | |||
| Centrohepatic | 1.231 | 0.529–2.865 | 0.630 | |||
| Deep behind a fictitious line of the portal axis | 0.875 | 0.209–3.662 | 0.855 | |||
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Variables | HR | 95% CI | p Value | HR | 95% CI |
| Dimension (mm) Subgroups | 1.057 | 0.999–1.118 | 0.054 | ||
| 10–20 (n; %) | 2.644 | 0.954–7.332 | 0.062 | ||
| 21–30 (n; %) | 0.518 | 0.148–1.814 | 0.303 | ||
| 31–40 (n; %) | 0.219 | 0.058–1.457 | 0.133 | ||
| Liver segment | |||||
| 1 (n; %) | -- | ||||
| 2 (n; %) | 1.484 | 0.516–4.262 | 0.464 | ||
| 3 (n; %) | 0.835 | 0.291–2.399 | 0.738 | ||
| 4 (n; %) | 0.658 | 0.203–2.134 | 0.486 | ||
| 5 (n; %) | 1.264 | 0.476–3.353 | 0.638 | ||
| 6 (n; %) | 0.400 | 0.119–1.350 | 0.140 | ||
| 7 (n; %) | 0.660 | 0.238–1.828 | 0.424 | ||
| 8 (n; %) | 1.879 | 0.834–4.235 | 0.128 | ||
| Location | |||||
| Anterior, superficial | 1.009 | 0.504–2.020 | 0.980 | ||
| Posterior, superficial | 0.680 | 0.280–1.653 | 0.394 | ||
| Centrohepatic | 1.128 | 0.488–2.608 | 0.779 | ||
| Deep behind a fictitious line of the portal axis | 1.905 | 0.436–8.331 | 0.392 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzulli, M.; Pecorelli, A.; Brandi, N.; Brocchi, S.; Tovoli, F.; Granito, A.; Carrafiello, G.; Ierardi, A.M.; Golfieri, R. The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool? J. Clin. Med. 2022, 11, 4399. https://doi.org/10.3390/jcm11154399
Renzulli M, Pecorelli A, Brandi N, Brocchi S, Tovoli F, Granito A, Carrafiello G, Ierardi AM, Golfieri R. The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool? Journal of Clinical Medicine. 2022; 11(15):4399. https://doi.org/10.3390/jcm11154399
Chicago/Turabian StyleRenzulli, Matteo, Anna Pecorelli, Nicolò Brandi, Stefano Brocchi, Francesco Tovoli, Alessandro Granito, Gianpaolo Carrafiello, Anna Maria Ierardi, and Rita Golfieri. 2022. "The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool?" Journal of Clinical Medicine 11, no. 15: 4399. https://doi.org/10.3390/jcm11154399
APA StyleRenzulli, M., Pecorelli, A., Brandi, N., Brocchi, S., Tovoli, F., Granito, A., Carrafiello, G., Ierardi, A. M., & Golfieri, R. (2022). The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool? Journal of Clinical Medicine, 11(15), 4399. https://doi.org/10.3390/jcm11154399