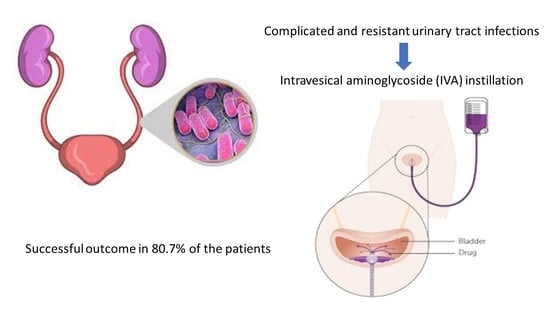
Are Intravesical Aminoglycosides the New Gold Standard in the Management of Refractory Urinary Tract Infection: A Systematic Review of Literature
Abstract
:1. Introduction
2. Methodology
- Studies reporting on the administration of intravesical aminoglycosides for the treatment and prevention of refractory UTIs.
- Articles written in the English language.
- All age groups, including pediatric studies.
- Studies with a minimal sample size of three patients.
- Exclusion criteria:
- Non-human studies, review articles, editorials, guidelines, and case reports.
- Studies with a non-UTI treatment indication.
- Studies reporting on non-aminoglycoside intravesical instillations.
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Outcomes of Interest
3. Results
3.1. Clinical Efficacy of IVA
3.2. Patient Compliance
3.3. Safety
4. Discussion
Limitations and Areas of Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pietropaolo, A.; Jones, P.; Moors, M.; Birch, B.; Somani, B.K. Use and Effectiveness of Antimicrobial Intravesical Treatment for Prophylaxis and Treatment of Recurrent Urinary Tract Infections (UTIs): A Systematic Review. Curr. Urol. Rep. 2018, 19, 78. Available online: https://pubmed.ncbi.nlm.nih.gov/30094687/ (accessed on 30 June 2022). [CrossRef]
- Van Nieuwkoop, C.; den Exter, P.L.; Elzevier, H.W.; den Hartigh, J.; van Dissel, J.T. Intravesical gentamicin for recurrent urinary tract infection in patients with intermittent bladder catheterisation. Int. J. Antimicrob. Agents 2010, 36, 485–490. Available online: https://pubmed.ncbi.nlm.nih.gov/20580533/ (accessed on 30 June 2022). [CrossRef] [PubMed]
- Abrams, P.; Hashim, H.; Tomson, C.; Macgowan, A.; Skews, R.; Warren, K. The use of intravesical gentamicin to treat recurrent urinary tract infections in lower urinary tract dysfunction. Neurourol. Urodyn. 2017, 36, 2109–2116. [Google Scholar] [CrossRef]
- Higgins, J.P.T. (Ed.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available online: http://handbook.cochrane.org;https://www.google.com/search?q=Higgins%2C+JPT%2C+Green+S+(editors).+Cochrane+handbook+for+systematic+reviews+of+interventions+Version+5.1.0+%5Bupdated+March+2011%5D.+The+Cochrane+Collaboration%2C+2011.+Available+from+http%3A%2F%2Fhandbook.cochrane.org.&o (accessed on 30 June 2022).
- Haldorson, A.M.; Keys, T.F.; Maker, M.D.; Opitz, J.L. Nonvalue of Neomycin Instillation after Intermittent Urinary Catheterization. Antimicrob. Agents Cheoterapy 1978, 14, 368–370. [Google Scholar] [CrossRef] [PubMed]
- McGuire, E.J.; Savastano, J.A. Treatment of intractable bacterial cystitis with intermittent catheterization and antimicrobial instillation: Case report. J. Urol. 1987, 137, 495–496. Available online: https://pubmed.ncbi.nlm.nih.gov/3820384/ (accessed on 30 June 2022). [CrossRef]
- Wan, J.; Kozminski, M.; Wang, S.C.; Faerber, G.J.; McGuire, E.J.; Bloom, D.A.; Ritchey, M.L. Intravesical instillation of gentamicin sulfate: In vitro, rat, canine, and human studies. Urology 1994, 43, 531–536. Available online: https://pubmed.ncbi.nlm.nih.gov/8154077/ (accessed on 30 June 2022). [CrossRef]
- Arap, M.P.P. Efficacy of Intermittent Intravesical Gentamicin Sulfate Solution for Recalcitrant Recurrent Cystitis in Women. Infect. Urol. 2003, 16. Available online: https://www.researchgate.net/publication/313168378_Efficacy_of_intermittent_intravesical_gentamicin_sulfate_solution_for_recalcitrant_recurrent_cystitis_in_women (accessed on 22 June 2022).
- Defoor, W.; Ferguson, D.; Mashni, S.; Creelman, L.; Reeves, D.; Minevich, E.; Reddy, P.; Sheldon, C. Safety of Gentamicin Bladder Irrigations in Complex Urological Cases. J. Urol. 2006, 175, 1861–1864. [Google Scholar] [CrossRef]
- Praba, B.A.; Utomo, T. Efficacy of intravesical instillation of netilmicin on managing uti. Indones J. Urol. 2015, 22. [Google Scholar] [CrossRef]
- Cox, L.; He, C.; Bevins, J.; Clemens, J.Q.; Stoffel, J.T.; Cameron, A.P. Gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on intermittent catheterization. Can. Urol. Assoc. J. 2017, 11, E350–E354. Available online: https://pubmed.ncbi.nlm.nih.gov/29382457/ (accessed on 30 June 2022). [CrossRef] [PubMed]
- Dray, E.V.; Clemens, J.Q. Recurrent urinary tract infections in patients with incomplete bladder emptying: Is there a role for intravesical therapy? Transl. Androl. Urol. 2017, 6 (Suppl. 2), S163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalenhoef, J.E.; Van Nieuwkoop, C.; Menken, P.H.; Bernards, S.T.; Elzevier, H.W.; Van Dissel, J.T. Intravesical Gentamicin Treatment for Recurrent Urinary Tract Infections Caused by Multidrug Resistant Bacteria. J. Urol. 2019, 201, 549–555. Available online: https://pubmed.ncbi.nlm.nih.gov/30316898/ (accessed on 30 June 2022). [CrossRef] [PubMed]
- Chernyak, S.; Salamon, C. Intravesical Antibiotic Administration in the Treatment of Recurrent Urinary Tract Infections: Promising Results From a Case Series. Female Pelvic. Med. Reconstr. Surg. 2020, 26, 152–154. Available online: https://pubmed.ncbi.nlm.nih.gov/31990805/ (accessed on 30 June 2022). [CrossRef]
- Marei, M.M.; Jackson, R.; Keene, D.J.B. Intravesical gentamicin instillation for the treatment and prevention of urinary tract infections in complex paediatric urology patients: Evidence for safety and efficacy. J. Pediatr. Urol. 2021, 17, e1–e65. [Google Scholar] [CrossRef] [PubMed]
- Pearman, J.W.; Bailey, M.; Harper, W.E.S. Comparison of the efficacy of “Trisdine” and kanamycin-colistin bladder instillations in reducing bacteriuria during intermittent catheterisation of patients with acute spinal cord trauma. Br. J. Urol. 1988, 62, 140–144. Available online: https://pubmed.ncbi.nlm.nih.gov/3136820/ (accessed on 15 June 2022). [CrossRef]
- Pearman, J.W. The value of kanamycin-colistin bladder instillations in reducing bacteriuria during intermittent catheterisation of patients with acute spinal cord injury. Br. J. Urol. 1979, 51, 367–374. Available online: https://pubmed.ncbi.nlm.nih.gov/533594/ (accessed on 30 June 2022). [CrossRef]
- Linsenmeyer, T.A.; Jain, A.; Thompson, B.W. Effectiveness of Neomycin/Polymyxin Bladder Irrigation to Treat Resistant Urinary Pathogens in Those with Spinal Cord Injury. J. Spinal Cord. Med. 1998, 22, 252–257. [Google Scholar] [CrossRef]
- Anderson, R.U. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J. Urol. 1980, 123, 364–366. Available online: https://pubmed.ncbi.nlm.nih.gov/6244414/ (accessed on 30 June 2022). [CrossRef]
- Waites, K.B.; Canupp, K.C.; Roper, J.F.; Camp, S.M.; Chen, Y. Evaluation of 3 Methods of Bladder Irrigation to Treat Bacteriuria in Persons With Neurogenic Bladder. J. Spinal. Cord. Med. 2006, 29, 217. [Google Scholar] [CrossRef]
- Rhame, F.S.; Perkash, I. Urinary tract infections occurring in recent spinal cord injury patients on intermittent catheterization. J. Urol. 1979, 122, 669–673. Available online: https://pubmed.ncbi.nlm.nih.gov/501823/ (accessed on 30 June 2022). [CrossRef]
- Huen, K.H.; Nik-Ahd, F.; Chen, L.; Lerman, S.; Singer, J. Neomycin-polymyxin or gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on clean intermittent catheterization. J. Pediatr. Urol. 2019, 15, e1–e178. Available online: https://pubmed.ncbi.nlm.nih.gov/30611650/ (accessed on 30 June 2022). [CrossRef] [PubMed]
- Block, M.B.D. Aminoglycosides. In StatPearls Treasure Island; The Royal Manchester Children’s Hospital, Manchester University NHS Foundation Trust: Manchester, UK, 2022. Available online: https://www.google.com/search?q=Block+M%252C+Blanchard+DL.+Aminoglycosides.+%255BUpdated+2021+Jul+23%255D.+In%253A+StatPearls+%255BInternet%255D.+Treasure+Island+(FL)%253A+StatPearls+Publishing%253B+2022+Jan-.+Available+from%253A+https%253A%252F%252Fwww.ncbi.nlm.nih.gov%252Fbooks%25 (accessed on 30 June 2022).
- Tyagi, P.; Wu, P.C.; Chancellor, M.; Yoshimura, N.; Huang, L. Recent advances in intravesical drug/gene delivery. Mol. Pharm. 2006, 3, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Study Design | No. of Patients | Mean Age | Male:Female | Additional Risk Factors/Comorbidities | Definition of Recurrent UTI in Included Studies |
|---|---|---|---|---|---|---|
| Haldorson et al., 1978 [5] | Prospective | 53 | ND | 33:20 | SCI *, multiple sclerosis, vascular disease, and cancer | Recurrent bacteriuria during intermittent catheterization |
| McGuire and Savastano 1987 [6] | Case series | 4 | 68 | 0:4 | Hemorrhagic cystitis, bladder dysfunction, and high residual volume | Failed response to oral antibiotic treatment |
| Wan et al., 1994 [7] | Prospective | 10 | 1–18 yo | 4:6 | Myelomeningocele, vesicoureteric reflux, augmentation enterocystoplasty, Crohn’s disease, and renal transplant | Positive urine cultures |
| Arap et al., 2003 [8] | Retrospective | 18 | 70 | 0:18 | Recurrent UTI | Failed response to oral antibiotic treatment |
| Defoor et al., 2006 [9] | Retrospective | 80 | 10 | 38:42 | Neurogenic bladder, bladder exstrophy, cloacal anomalies, Hinman syndrome, vesicoureteral reflux, hypospadias, posterior urethral valves, bladder reconstruction, and renal transplantation | Failed response to oral antibiotic treatment |
| Praba and Utomo 2015 [10] | Prospective clinical trial | 28 | 58.9 | 54:2 | Benign prostatic hyperplasia and prostate carcinoma | Nosocomial catheter-associated UTIs |
| Abrams et al., 2017 [3] | Retrospective | 27 | 55 | 7:20 | Neobladders, neurogenic bladder, ileocystoplasty, and ISC ** | Recurrent UTI, failed oral prophylaxis |
| Cox et al., 2017 [11] | Prospective | 22 | 37.5 | 22:15 | SCI, multiple sclerosis, and myelodysplasia transverse myelitis | Four UTIs in the preceding 6-month period |
| Dray VE et al., 2017 [12] | Retrospective | 22 | ND | ND | ISC | Three or greater urinary tract infections (UTIs) in one year, or two or more UTIs in six months |
| Stalenhoef et al., 2019 [13] | Retrospective | 63 | 61 | 51:12 | ISC, chronic bacterial prostatitis, vesicoureteral reflux, and neobladder | Recurrent UTIs despite failed oral prophylaxis |
| Chernyak and Salamon 2020 [14] | Retrospective case series | 12 | 80.3 | 00:12 | Neurogenic bladder and structurally abnormal urinary tract | Two UTIs in 6 months or three UTIs in a 1 year period |
| Marei et al., 2021 [15] | Retrospective | 24 | 3.8 | 13:11 | Neurogenic bladder, bladder exstrophy, cloacal anomalies, and posterior urethral valves | Recurrent UTIs despite failed oral prophylaxis |
| Study | Study Design | No. of Patients | Mean Age | Male:Female | Additional Risk Factors/Comorbidities | Definition of Recurrent UTI |
|---|---|---|---|---|---|---|
| Pearman et al., 1988 [16] | Prospective randomized comparative | 18 | 26 | 18:0 | SCI | Consecutive positive urinary cultures |
| Pearman 1979 [17] | Randomized control trial | 22 | 30 | 17:5 | SCI | Consecutive positive urinary cultures |
| Linsenmeyer et al., 1998 [18] | Retrospective | 12 | ND | 7:3 | SCI | Culture-positive UTIs unable to be managed with oral antibiotics |
| Anderson 1980 [19] | Randomized prospective | 17 | ND | 64:0 | Acute neurogenic bladder | Significant bacteriuria |
| Waites et al., 2006 [20] | Randomized | 30 | ND | ND | SCI and neurogenic bladder | Recurrent microscopic bacteriuria and pyuria |
| Rhame and Perkash 1979 [21] | Retrospective | 70 | 36.4 | 70:0 | SCI | Positive urine culture |
| Huen et al., 2019 [22] | Retrospective | 52 | 14.5 | 21:31 | Spina bifida, cloacal exstrophy, posterior urethral valves, appendicovesicostomy, and enterocystoplasty | Frequent symptomatic UTIs despite oral antibiotic prophylaxis |
| Study | Intervention | Follow-Up (Range) | Successful Outcome ** | Discontinued Treatment | Side Effects | Serum Level | Change in Sensitivities | Developed New Resistance |
|---|---|---|---|---|---|---|---|---|
| Haldorson et al., 1978 [5] | 0.1% neomycin solution | 6 weeks | 25/53 | 0 | ND | ND | ND | 37/53 |
| McGuire and Savastano 1987 [6] | 20 mL of 240 mg gentamicin in 1 L 0.9% NaCl * | 20 months (3–36 months) | 4/4 | 0 | ND | ND | ND | ND |
| Wan et al., 1994 [7] | 30–60 mL 480mg gentamicin in 1 L 0.9% NaCl | 1 week | 10/10 | 0 | 0 | Yes, negligible | ND | ND |
| Arap et al., 2003 [8] | 480 mg gentamicin in 1 L 0.9% NaCl + 100 mL sodium carbonate | 65.1 months | 12/18 | 3/18 | 3 UTI | Yes, negligible | 0 | 3/18 |
| Defoor et al., 2006 [9] | 0.48 mg/mL gentamicin in 30 mL 0.9% NaCl | 90 days | 75/80 | Variable not trackable | Minor rise in serum creatinine for three patients with chronic renal insufficiency | Yes, negligible | 16/80 | 5/80 |
| Praba and Utomo 2015 [10] | 25 mg/1 mL netilmicin in 50 mL 0.9% NaCl | 4 days | 22/28 | 0 | ND | ND | ND | ND |
| Abrams et al., 2017 [3] | 80 mg gentamicin in 10 mL 0.9% NaCl | 26 months (2–67) | 22/27 | 6/27 | 0 | Yes, negligible | 18/27 | 1/27 |
| Cox et al., 2017 [11] | 14.4–28.8 mg gentamicin in 30–60 mL of 0.9% NaCl (according to bladder capacity) | 6 weeks | 22/22 | 0 | 1 yeast infection, 1 diarrhea | Not checked | 9/22 | 8/22 |
| Dray VE et al., 2017 [12] | 14.4–28.8 mg gentamicin in 30–60 mL of 0.9% NaCl (according to bladder capacity) | ND | 22/22 | ND | 0 | Yes, negligible | ND | ND |
| Stalenhoef et al., 2019 [13] | 80 mg gentamicin in 20 mL of 0.9% NaCl | 42 weeks (6–148) | 52/63 | 10 | Hearing loss (n = 2), vaginal discomfort (n = 10), and abdominal discomfort (n = 3) | Yes, negligible | 4/14 | 6/63 |
| Chernyak and Salamon 2020 [14] | 80 mg gentamicin in 60 mL 0.9% NaCl or 80 mg tobramycin in 100 mL 0.9% NaCl | 6 months | 12/12 | 0 | 0 | Not checked | 8/12 | 0/12 |
| Marei et al., 2021 [15] | 8 mg gentamicin in 20 mL 0.9% NaCl or 20 mg Gent in 50 mL 0.9% NaCl (per bladder capacity) | 3 years | 11/19 | 1 | ND | One detectable | ND | 1/24 |
| Study | Intervention | Follow-Up | Successful Outcome ** | Discontinued Treatment | Side Effects | Serum Level | Change in Sensitivities | Developed New Resistance |
|---|---|---|---|---|---|---|---|---|
| Pearman et al., 1988 [16] | 150 mg kanamycin + 30 mg colistin in 25 mL sterile water | 130 days | 2/7 | ND | 0 | ND | ND | ND |
| Pearman 1979 [17] | kanamycin 150 mg + colistin 30 mg in 25 mL sterile water | 120 days | 9/17 | ND | ND | ND | ND | ND |
| Linsenmeyer et al., 1998 [18] | 30 mL neomycin/polymyxin solution | 6 months | 9/12 | 2/12 | Allergy | ND | 9/12 | 3/12 |
| Anderson 1980 [19] | 30 mL 160 mg neomycin, 800,000 polymyxin-B in sterile water | ND | 17/17 | ND | ND | ND | ND | ND |
| Waites et al., 2006 [20] | 30 of 40 mg/mL neomycin sulfate and 200,000 units/mL polymyxin B | 8 weeks | 23/30 | 8/30 | Autonomic dysreflexia (n = 2) | ND | 12/30 | 18/30 |
| Rhame and Perkash 1979 [21] | 50 mL of 120 ug/mL neomycin and 60 ug/mL polymyxin B | 28 months | 51/70 | ND | ND | ND | 1/70 | ND |
| Huen et al., 2019 [22] | 30–50 mL 480 mg gentamicin in 1 L 0.9% NaCl | 6 months | 52/52 | 6/52 | ND | ND | 14/52 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, A.; Pietropaolo, A.; Brown, G.; Somani, B.K. Are Intravesical Aminoglycosides the New Gold Standard in the Management of Refractory Urinary Tract Infection: A Systematic Review of Literature. J. Clin. Med. 2022, 11, 5703. https://doi.org/10.3390/jcm11195703
Ong A, Pietropaolo A, Brown G, Somani BK. Are Intravesical Aminoglycosides the New Gold Standard in the Management of Refractory Urinary Tract Infection: A Systematic Review of Literature. Journal of Clinical Medicine. 2022; 11(19):5703. https://doi.org/10.3390/jcm11195703
Chicago/Turabian StyleOng, Andrea, Amelia Pietropaolo, George Brown, and Bhaskar K. Somani. 2022. "Are Intravesical Aminoglycosides the New Gold Standard in the Management of Refractory Urinary Tract Infection: A Systematic Review of Literature" Journal of Clinical Medicine 11, no. 19: 5703. https://doi.org/10.3390/jcm11195703
APA StyleOng, A., Pietropaolo, A., Brown, G., & Somani, B. K. (2022). Are Intravesical Aminoglycosides the New Gold Standard in the Management of Refractory Urinary Tract Infection: A Systematic Review of Literature. Journal of Clinical Medicine, 11(19), 5703. https://doi.org/10.3390/jcm11195703