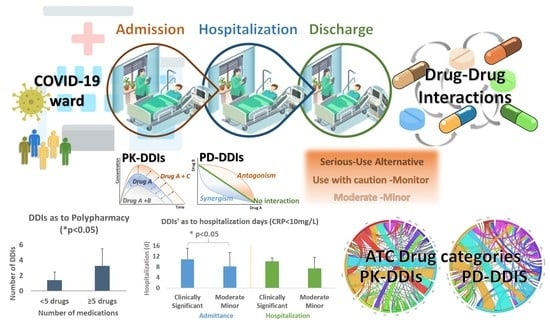
Drug-Drug Interactions among Patients Hospitalized with COVID-19 in Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Approval
2.2. Evaluation of DDIs
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics, Comorbidities, and Clinical Status
3.2. Drugs Administered to COVID-19 Patients
3.3. DDIs, Clinical Significance, and Related Pharmacological Mechanisms
3.4. Impact of Polypharmacy and DDIs on the Hospitalization Status of COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ATC-Codes | Drug Category | Pharmacological Subgroup |
|---|---|---|
| A02 | Drugs for acid-related disorders | Proton pump inhibitors |
| A10 | Drugs used in diabetes | Biguanides; dipeptidyl peptidase 4 (DPP-4) inhibitors; sodium-glucose co-transporter 2 (SGLT2) inhibitors |
| A11 | Vitamins | Vitamin B-complex |
| A12 | Mineral supplements | Calcium, combinations with vitamin D; potassium; magnesium |
| B01 | Antithrombotic agents | Vitamin-K antagonists; heparin group; direct oral anticoagulants |
| B03 | Antianemic preparations | Ferrous supplements |
| C01 | Cardiac therapy | Cardiac glycosides; antiarrhythmics; Vasodilators |
| C02 | Antihypertensives | Imidazoline receptor agonists |
| C03 | Diuretics | Thiazides; sulfonamides; aldosterone antagonists |
| C07 | β-blockers | selective β-blockers, α-and β-blockers |
| C08 | Ca2+ channel blockers (CCBs) | CCBs with vascular effects; CCBs with direct cardiac effects |
| C09 | Agents acting on the renin–angiotensin system | ACE inhibitors; ARBs |
| C10 | Lipid modifying agents | HMG CoA reductase inhibitors (statins) |
| G03 | Sex hormones and modulators of the genital system | Progestogens and estrogens |
| G04 | Urologicals | Drugs used in benign prostatic hypertrophy |
| H02 | Corticosteroids for systemic use | Glucocorticoids |
| H03 | Thyroid therapy | Thyroid hormones |
| J01 | Antibacterials for systemic use | Penicillins; macrolides; quinolones |
| J02 | Antimycotics for systemic use | Imidazole and triazole derivatives |
| J05 | Antivirals for systemic use | Direct acting antiviral drugs (remdesivir) |
| L01 | Antineoplastic agents | Protein kinase inhibitors |
| L02 | Endocrine therapy | Gonadotropin-releasing hormone analogs |
| L04 | Immunosuppressants | Interleukin inhibitors; antimetabolites |
| M04 | Antigout preparations | Uric acid inhibitors |
| N02 | Analgesics | Analgesics and antipyretics |
| N03 | Antiepileptics | Carboxamide (carbamazepine) and fatty acid (valproic acid) derivatives |
| N04 | Anti-Parkinson drugs | Dopaminergic agents |
| N05 | Psycholeptics | Antipsychotics, anxiolytics, and sedatives |
| N06 | Psychoanaleptics | Antidepressants |
| R03 | Drugs for obstructive airway diseases | β-2-receptor agonists; glucocorticoids |
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biancolella, M.; Colona, V.L.; Mehrian-Shai, R.; Watt, J.L.; Luzzatto, L.; Novelli, G.; Reichardt, J.K.V. COVID-19 2022 update: Transition of the pandemic to the endemic phase. Hum. Genom. 2022, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Whitaker, M.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Alden, N.B.; Yousey-Hindes, K.; Anderson, E.J.; et al. COVID-19-Associated Hospitalizations Among Adults During SARS-CoV-2 Delta and Omicron Variant Predominance, by Race/Ethnicity and Vaccination Status—COVID-NET, 14 States, July 2021–January 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Jassat, W.; Abdool Karim, S.S.; Mudara, C.; Welch, R.; Ozougwu, L.; Groome, M.J.; Govender, N.; von Gottberg, A.; Wolter, N.; Wolmarans, M.; et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: A retrospective observational study. Lancet Glob. Health 2022, 10, e961–e969. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative Analysis of the Risks of Hospitalisation and Death Associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants in England. SSRN Electron. J. 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Chi, J.; Dong, B.; Lv, W.; Shen, L.; Wang, Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 99, 47–56. [Google Scholar] [CrossRef]
- Du, P.; Li, D.; Wang, A.; Shen, S.; Ma, Z.; Li, X. A Systematic Review and Meta-Analysis of Risk Factors Associated with Severity and Death in COVID-19 Patients. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6660930. [Google Scholar] [CrossRef]
- Vahey, G.M.; McDonald, E.; Marshall, K.; Martin, S.W.; Chun, H.; Herlihy, R.; Tate, J.E.; Kawasaki, B.; Midgley, C.M.; Alden, N.; et al. Risk factors for hospitalization among persons with COVID-19—Colorado. PLoS ONE 2021, 16, e0256917. [Google Scholar] [CrossRef]
- Molani, S.; Hernandez, P.V.; Roper, R.T.; Duvvuri, V.R.; Baumgartner, A.M.; Goldman, J.D.; Ertekin-Taner, N.; Funk, C.C.; Price, N.D.; Rappaport, N.; et al. Risk factors for severe COVID-19 differ by age for hospitalized adults. Sci. Rep. 2022, 12, 6568. [Google Scholar] [CrossRef]
- Hergens, M.P.; Bell, M.; Haglund, P.; Sundström, J.; Lampa, E.; Nederby-Öhd, J.; Östlund, M.R.; Cars, T. Risk factors for COVID-19-related death, hospitalization and intensive care: A population-wide study of all inhabitants in Stockholm. Eur. J. Epidemiol. 2022, 37, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.H.; Tipih, T.; Makoah, N.A.; Vermeulen, J.G.; Goedhals, D.; Sempa, J.B.; Burt, F.J.; Taylor, A.; Mahalingam, S. Comorbidities in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. MBio 2021, 12, e03647-20. [Google Scholar] [CrossRef] [PubMed]
- Bajgain, K.T.; Badal, S.; Bajgain, B.B.; Santana, M.J. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am. J. Infect. Control 2021, 49, 238–246. [Google Scholar] [CrossRef]
- Preskorn, S.H.; Quadri, S. Why Are Patients With COVID-19 at Risk for Drug-Drug Interactions? J. Psychiatr. Pract. 2020, 26, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Classifying drug interactions. Br. J. Clin. Pharmacol. 2004, 58, 343–344. [Google Scholar] [CrossRef] [Green Version]
- Kohler, G.I.; Bode-Boger, S.M.; Busse, R.; Hoopmann, M.; Welte, T.; Boger, R.H. Drug-drug interactions in medical patients: Effects of in-hospital treatment and relation to multiple drug use. Int. J. Clin. Pharmacol. Ther. 2000, 38, 504–513. [Google Scholar] [CrossRef]
- Dechanont, S.; Maphanta, S.; Butthum, B.; Kongkaew, C. Hospital admissions/visits associated with drug-drug interactions: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2014, 23, 489–497. [Google Scholar] [CrossRef]
- Spanakis, M.; Patelarou, A.; Patelarou, E.; Tzanakis, N. Drug Interactions for Patients with Respiratory Diseases Receiving COVID-19 Emerged Treatments. Int. J. Environ. Res. Public Health 2021, 18, 11711. [Google Scholar] [CrossRef]
- Conti, V.; Sellitto, C.; Torsiello, M.; Manzo, V.; De Bellis, E.; Stefanelli, B.; Bertini, N.; Costantino, M.; Maci, C.; Raschi, E.; et al. Identification of Drug Interaction Adverse Events in Patients With COVID-19: A Systematic Review. JAMA Netw. Open 2022, 5, e227970. [Google Scholar] [CrossRef]
- Rezaee, H.; Pourkarim, F.; Pourtaghi-Anvarian, S.; Entezari-Maleki, T.; Asvadi-Kermani, T.; Nouri-Vaskeh, M. Drug-drug interactions with candidate medications used for COVID-19 treatment: An overview. Pharmacol. Res. Perspect. 2021, 9, e00705. [Google Scholar] [CrossRef]
- Baburaj, G.; Thomas, L.; Rao, M. Potential Drug Interactions of Repurposed COVID-19 Drugs with Lung Cancer Pharmacotherapies. Arch. Med. Res. 2021, 52, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, N.; Talebi, A.; Moghadamnia, M.; Taloki, Z.N.; Shakiba, A. Drug interactions of psychiatric and COVID-19 medications. Basic Clin. Neurosci. 2020, 11, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Karsidag, S.; Cinar, N.; Ates, M.F.; Demir, S.; Eren, F.; Neyal, A.; Kisabay Ak, A.; Bora Tokcaer, A.; Erkoc Ataoglu, E.; et al. The Impact of the COVID-19 Lockdown on the Quality of Life in Chronic Neurological Diseases: The Results of a COVQoL-CND Study. Eur. Neurol. 2021, 84, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Nelson, S.D.; Su, Y.; Malin, B.; Li, L.; Chen, Y. Detecting drug-drug interactions between therapies for COVID-19 and concomitant medications through the FDA adverse event reporting system. Front. Pharmacol. 2022, 13, 2853. [Google Scholar] [CrossRef]
- Larkin, H.D. Paxlovid Drug Interaction Screening Checklist Updated. JAMA 2022, 328, 1290. [Google Scholar] [CrossRef]
- Hoffmann-La Roche Limited. Casirivimab and Imdevimab for Injection-Product Monograph; Hoffmann-La Roche Limited: Mississauga, ON, Canada, 2021. [Google Scholar]
- GlaxoSmithKline. Xevudy-Annex i Summary of Product Characteristics; GlaxoSmithKline Trading Services Limited: Dublin, Ireland, 2022. [Google Scholar]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef] [Green Version]
- Spanakis, M.; Melissourgaki, M.; Lazopoulos, G.; Patelarou, A.E.; Patelarou, E. Prevalence and clinical significance of drug–drug and drug–dietary supplement interactions among patients admitted for cardiothoracic surgery in greece. Pharmaceutics 2021, 13, 239. [Google Scholar] [CrossRef]
- Spanakis, M.; Roubedaki, M.; Tzanakis, I.; Zografakis-Sfakianakis, M.; Patelarou, E.; Patelarou, A. Impact of adverse drug reactions in patients with end stage renal disease in Greece. Int. J. Environ. Res. Public Health 2020, 17, 9101. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Ajimura, C.M.; Jagan, N.; Morrow, L.E.; Malesker, M.A. Drug Interactions with Oral Inhaled Medications. J. Pharm. Technol. 2018, 34, 273. [Google Scholar] [CrossRef]
- Robinson, P.C.; Liew, D.F.L.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.P.; Hussell, T.; et al. COVID-19 therapeutics: Challenges and directions for the future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Azoulay, E.; Al-Dorzi, H.M.; Phua, J.; Salluh, J.; Binnie, A.; Hodgson, C.; Angus, D.C.; Cecconi, M.; Du, B.; et al. How the COVID-19 pandemic will change the future of critical care. Intensive Care Med. 2021, 47, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Iloanusi, S.; Mgbere, O.; Essien, E.J. Polypharmacy among COVID-19 patients: A systematic review. J. Am. Pharm. Assoc. 2021, 61, e14–e25. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, G.; Brilli, V.; Lanzi, C.; Burgalassi, A.; Ieri, A.; Bonaiuti, R.; Romano, E.; Innocenti, R.; Mannaioni, G.; Vannacci, A.; et al. Adverse drug reactions in SARS-CoV-2 hospitalised patients: A case-series with a focus on drug–drug interactions. Intern. Emerg. Med. 2021, 16, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.S.; Sehayek, D.; Gabrielli, S.; Zhang, X.; McCusker, C.; Ben-Shoshan, M. COVID-19 and comorbidities: A systematic review and meta-analysis. Postgrad. Med. 2020, 132, 749–755. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA—J. Am. Med. Assoc. 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Zaman, M.; Khan, W.A.; Rajaram, S.S. Comorbidities and COVID-19. In COVID-19 by Cases: A Pandemic Review; British Medical Journal Publishing Group: London, UK, 2021; Volume 377, pp. 283–294. ISBN 9781685072629. [Google Scholar]
- Sharifpour, M.; Rangaraju, S.; Liu, M.; Alabyad, D.; Nahab, F.B.; Creel-Bulos, C.M.; Jabaley, C.S. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS ONE 2020, 15, e0242400. [Google Scholar] [CrossRef]
- Braz-de-Melo, H.A.; Faria, S.S.; Pasquarelli-do-Nascimento, G.; Santos, I.D.O.; Kobinger, G.P.; Magalhães, K.G. The Use of the Anticoagulant Heparin and Corticosteroid Dexamethasone as Prominent Treatments for COVID-19. Front. Med. 2021, 8, 615333. [Google Scholar] [CrossRef]
- NIH. COVID-19 Treatment Guidelines 2. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 3 August 2022).
- National Public Health Organization EOODY COVID-19 Guidelines. Available online: https://eody.gov.gr/wp-content/uploads/2022/02/covid_19_algorithmos-nosileuomenon_20220217.pdf (accessed on 11 September 2022).
- Cattaneo, D.; Pasina, L.; Maggioni, A.P.; Oreni, L.; Conti, F.; Pezzati, L.; Casalini, G.; Bonazzetti, C.; Morena, V.; Ridolfo, A.; et al. Drug–Drug Interactions and Prescription Appropriateness at Hospital Discharge: Experience with COVID-19 Patients. Drugs Aging 2021, 38, 341–346. [Google Scholar] [CrossRef]
- Khiali, S.; Entezari-Maleki, T. Anticoagulation in COVID-19: DDI Perspective. Clin. Appl. Thromb. 2020, 26, 1076029620959457. [Google Scholar] [CrossRef]
- Laine, L.; Hennekens, C. Proton pump inhibitor and clopidogrel interaction: Fact or fiction. Am. J. Gastroenterol. 2010, 105, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gjestad, C.; Westin, A.A.; Skogvoll, E.; Spigset, O. Effect of proton pump inhibitors on the serum concentrations of the selective serotonin reuptake inhibitors citalopram, escitalopram, and sertraline. Ther. Drug Monit. 2015, 37, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiśniowska, B.; Tylutki, Z.; Wyszogrodzka, G.; Polak, S. Drug-drug interactions and QT prolongation as a commonly assessed cardiac effect—comprehensive overview of clinical trials. BMC Pharmacol. Toxicol. 2016, 17, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatsisvili, A.; Sapounidis, I.; Pavlidou, G.; Zoumpouridou, E.; Karakousis, V.-A.A.; Spanakis, M.; Teperikidis, L.; Niopas, I. Potential drug–drug interactions in prescriptions dispensed in community pharmacies in Greece. Pharm. World Sci. 2010, 32, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulou, E.; Kontogiorgis, C.; Nena, E.; Constantinides, T.; Kolios, G. The complex phenomenon of polypharmacy in older age people of Greece: Data from the new era of e-prescribing. Drugs Ther. Perspect. 2017, 33, 580–584. [Google Scholar] [CrossRef]
- Andersson, M.L.; Böttiger, Y.; Kockum, H.; Eiermann, B. High Prevalence of Drug–Drug Interactions in Primary Health Care is Caused by Prescriptions from other Healthcare Units. Basic Clin. Pharmacol. Toxicol. 2018, 122, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Manias, E.; Kusljic, S.; Wu, A. Interventions to reduce medication errors in adult medical and surgical settings: A systematic review. Ther. Adv. Drug Saf. 2020, 11. [Google Scholar] [CrossRef]
- Perez, M.; Masse, M.; Deldicque, A.; Beuscart, J.B.; De Groote, P.; Desbordes, J.; Fry, S.; Musy, E.; Odou, P.; Puisieux, F.; et al. Analysis of clinical pharmacist interventions in the COVID-19 units of a French university hospital. Eur. J. Hosp. Pharm. 2021, 29, e30–e35. [Google Scholar] [CrossRef]
- Guthrie, B.; Makubate, B.; Hernandez-Santiago, V.; Dreischulte, T. The rising tide of polypharmacy and drug-drug interactions: Population database analysis 1995-2010. BMC Med. 2015, 13, 74. [Google Scholar] [CrossRef] [Green Version]
- Cantudo-Cuenca, M.D.; Gutiérrez-Pizarraya, A.; Pinilla-Fernández, A.; Contreras-Macías, E.; Fernández-Fuertes, M.; Lao-Domínguez, F.A.; Rincón, P.; Pineda, J.A.; Macías, J.; Morillo-Verdugo, R. Drug-drug interactions between treatment specific pharmacotherapy and concomitant medication in patients with COVID-19 in the first wave in Spain. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Brandariz-Nuñez, D.; Correas-Sanahuja, M.; Guarc, E.; Picón, R.; García, B.; Gil, R. Potential drug-drug interactions in COVID 19 patients in treatment with lopinavir/ritonavir. Med. Clin. 2020, 155, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Manjhi, P.K.; Kumar, R.; Priya, A.; Rab, I. Drug-Drug Interactions in Patients with COVID-19: A Retrospective Study at a Tertiary Care Hospital in Eastern India. Maedica 2021, 16, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Armahizer, M.J.; Seybert, A.L.; Smithburger, P.L.; Kane-Gill, S.L. Drug-drug interactions contributing to QT prolongation in cardiac intensive care units. J. Crit. Care 2013, 28, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Vieweg, W.V.R. New Generation Antipsychotic Drugs and QTc Interval Prolongation. Prim. Care Companion J. Clin. Psychiatry 2003, 05, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Clift, A.K.; Coupland, C.A.C.; Keogh, R.H.; Diaz-Ordaz, K.; Williamson, E.; Harrison, E.M.; Hayward, A.; Hemingway, H.; Horby, P.; Mehta, N.; et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: National derivation and validation cohort study. BMJ 2020, 371, m3731. [Google Scholar] [CrossRef]
- Nafilyan, V.; Humberstone, B.; Mehta, N.; Diamond, I.; Coupland, C.; Lorenzi, L.; Pawelek, P.; Schofield, R.; Morgan, J.; Brown, P.; et al. An external validation of the QCovid risk prediction algorithm for risk of mortality from COVID-19 in adults: A national validation cohort study in England. Lancet. Digit. Health 2021, 3, e425–e433. [Google Scholar] [CrossRef]
- Saber-Moghaddam, N.; Hejazi, S.; Elyasi, S. Potential Drug–Drug Interactions among Hospitalized COVID-19 Patients Admitted to Medical Wards of a Referral Hospital, North-East of Iran: A Cross Sectional Study. J. Pharm. Care 2021, 9, 88–95. [Google Scholar] [CrossRef]
- Moura, C.; Prado, N.; Acurcio, F. Potential drug-drug interactions associated with prolonged stays in the intensive care unit: A retrospective cohort study. Clin. Drug Investig. 2011, 31, 309–316. [Google Scholar] [CrossRef]
- ECDC Weekly Epidemiological Update: Omicron Variant of Concern (VOC)—Week 1 (data as of 7 January 2022) EU/EEA. Available online: https://www.ecdc.europa.eu/en/news-events/weekly-epidemiological-update-omicron-variant-concern-voc-week-2-data-20-january-2022 (accessed on 15 September 2022).





| Methods | |
|---|---|
| Study design | Observational, retrospective, and descriptive study of DDIs in patients admitted with COVID-19 |
| Setting | COVID-19 ward, University Hospital Heraklion, Crete, Greece |
| Participants | Patients requiring inpatient treatment for COVID-19 |
| Variables | Record of demographic characteristics; clinical values; comorbidities; medication regimens; number of DDIs; clinical significance; hospitalization days |
| Data sources/ measurement | DDIs are based on literature searches and relative databases (Medscape, drugs.com, accessed on 1 April–30 July 2022) |
| Study size | Target population: patients admitted with COVID-19 Study population: signed informed consent form |
| Bias | Diligence in informing the purpose and objectives of the study Diligence in recording the medication regimens in the correct time periods Recording demographics and medication regimens Analysis of data regarding the significance |
| Results | |
| Participants | The informed consent form was signed by 125 participants (76 males/49 females) |
| Descriptive data | Average comorbidities: 4.0 Average hospitalization days: 8.6 (median 7) Admittance: laboratory confirmation for SARS-CoV-2 Vaccination status: 52% complete, 15% partial, and 23% none Mortality: 13% |
| Outcome data | Comorbidities: Cardiovascular disorders (58.4%) and diabetes (types I and II) (29.6%) 226 unique DDIs PK-DDIs: 32.0% and PD-DDIs: 68.0% |
| Main results | Patients with at least 1 potential DDI: 67.2% (admission), 92.8% (hospitalization), and 60% (discharge) Clinically significant DDIs: 40.3% (admission), 21% (hospitalization), and 40.7% (discharge) Patients with comorbidities had an increased number of DDIs (p < 0.05, 95% CI) DDIs were more prevalent for patients in a polypharmacy state (p < 0.05, 95% CI) Exponential correlation between DDIs and number of drugs Clinically significant DDIs were observed in patients that also had prolonged hospitalization (p < 0.05, 95% CI) |
| Demographics | Mean (±Standard Deviation) | Min/Max |
|---|---|---|
| Age (y) | 72.5 (±14.7) | 33/97 |
| Height (m) | 1.7 (±0.2) | 1.2/1.9 |
| Weight (kg) | 81.3 (±19.2) | 48.0/130.0 |
| Body Mass Index (BMI, kg/m2) | 33.1 (±9.1) | 22.2/50.0 |
| Comorbidities | 4 (±3) | 0/12 |
| Vaccination | 5% (3 doses); 14.4% (2 doses); 81.4% (1 or no dose) | |
| Duration of hospitalization (d) | 8.6 (±4.7) (median = 7) | 2/74 |
| Mortality | 13% (13% fully; 1% partially and 20% unvaccinated) | |
| Polypharmacy (≥5 drugs) | Admission = 55.2%; Hospitalization = 82.0%; Discharge = 55.8% | |
| Residence & Social Habits | ||
| Urban | 65 (52%) | |
| Suburban | 9 (7%) | |
| Semi-urban | 14 (11%) | |
| Rural | 35 (28%) | |
| Smoking | 38 (30%) | |
| Mechanisms of PK-DDIs | N |
|---|---|
| Inhibition of CYP-mediated metabolism | 39 |
| Reduced bioavailability due to pH-dependent solubility | 23 |
| Reduced metabolism (non-CYP) | 21 |
| Dual inhibition of CYP metabolism, P-gp, or other proteins transport | 13 |
| Increased serum urate and Ct of metabolite (oxypurinol) | 11 |
| Inhibition of P-gp-mediated transport | 10 |
| Induction of CYP-mediated metabolism | 10 |
| Modulation of GI absorption | 4 |
| Inhibition of influx-mediated transport (e.g., OAT1B1 or OCT2) | 3 |
| Renal tubular clearance | 2 |
| Protein binding competition | 2 |
| Dual induction of CYP-mediated metabolism and P-gp transport | 2 |
| Restore suppressed CYP expression caused by inflammation | 1 |
| Decrease tubular secretion | 1 |
| Mechanisms of PD-DDIs | |
| Modulation of anticoagulation action and altered INR-monitor | 183 |
| QT prolongation | 63 |
| Risk for hyperkalemia | 25 |
| Risk of tendon rupture | 24 |
| Risk for hypoglycemia | 18 |
| GI side effects | 16 |
| Deterioration in renal function (elderly) | 11 |
| PD antagonism-acute bronchospasm | 11 |
| PD synergism, sedation, and respiratory depression | 9 |
| PD antagonism-altered antihypertensive response | 7 |
| Risk for hypokalemia | 7 |
| Risk for hyperglycemia | 6 |
| Reduce renal function and antihypertensive effect of ACE inhibitors | 4 |
| Risk for serotonin syndrome | 4 |
| PD synergism-hypotensive effects | 4 |
| Risk for hyponatremia | 3 |
| Additive anticholinergic effects | 3 |
| PD-antagonism decreased effect of levodopa | 2 |
| Hypotension with hyperglycemia | 2 |
| PD-synergism cardiovascular side effects | 2 |
| Quinolone administration may result in hyper- or hypoglycemia | 2 |
| Risk for nephrotoxicity and/or ototoxicity. | 1 |
| PD-antagonism of Ca2+ with Ca2+ channel blockers | 1 |
| PD-synergism and excessive parasympatholytic effects | 1 |
| PD-synergism increased risk for serious infection | 1 |
| Drug A | Drug B | ATC | Pharmacological Outcome | Significance | N | |
|---|---|---|---|---|---|---|
| Acenocoumarol | Methylprednisolone | B01 | H02 | PD-INR-monitor | Monitor | 3 |
| Remdesivir | J05 | Monitor | 3 | |||
| Ceftriaxone | J01 | SUA | 2 | |||
| Rosuvastatin | A02 | Moderate | 2 | |||
| Esomeprazole | C10 | PK-CYP inhibition | Moderate | 3 | ||
| Allopurinol | Furosemide | M04 | C03 | PK-Ct metabolite | Monitor | 11 |
| Amiodarone | Metformin | C01 | A10 | PK-renal clearance | Moderate | 2 |
| Aspirin | Valsartan, Telmisartan | N02 | C09 | PD-Renal function (elderly) | Moderate | 7 |
| Ramipril | Moderate | 3 | ||||
| Azithromycin | Mirtazapine | J01 | N06 | PD-QT prolongation | Monitor | 2 |
| Carvedilol | Dabigatran | C07 | B01 | PK-P-gp inhibition | Monitor | 2 |
| Clopidogrel | Esomeprazole, Omeprazole | B01 | A02 | PK-CYP inhibition | SUA | 10 |
| Dexamethasone | Levofloxacin, Ciprofloxacin | H02 | J01 | PD-Risk of tendon rupture | Moderate | 16 |
| Digoxin | Esomeprazole | C01 | A02 | PK-P-gp inhibition | Monitor | 2 |
| Azithromycin | C01 | J01 | Monitor | 2 | ||
| Diltiazem | Rivaroxaban | C08 | B01 | PK-CYP, P-gp inhibition | Monitor | 2 |
| Enoxaparin | Dabigatran | B01 | B01 | PD-INR-monitor | SUA | 2 |
| Dexamethasone, Methylprednisolone | H02 | Moderate | 91 | |||
| Budesonide | R03 | Moderate | 28 | |||
| Azithromycin Piperacilin Ceftaroline | J01 | Moderate | 21 | |||
| Moderate | 12 | |||||
| Moderate | 6 | |||||
| Citalopram | C09 | Moderate | 4 | |||
| Irbesartan, Telmisartan | C09 | PD-hyperkalemia | Moderate | 6 | ||
| Ramiprin | N06 | Moderate | 5 | |||
| Escitalopram | Esomeprazole | N06 | A02 | PK-CYP inhibition | Monitor | 7 |
| Leuprolide | L02 | QT prolongation | SUA | 3 | ||
| Gliclazide | Furosemide | A10 | C03 | PD-hyperglycemia | Moderate | 2 |
| Aspirin | N02 | PD-hypoglycemia | Moderate | 2 | ||
| Haloperidol | Quetiapine | N05 | N05 | PD-QT prolongation | Monitor | 3 |
| Indacaterol | Formoterol | R03 | C07 | PD-acute bronchospasm | Moderate | 2 |
| Insulin | Levofloxacin | A10 | J01 | PD-blood glucose | Monitor | 2 |
| Ipratropium | Quetiapine | R03 | N05 | PD-hypoglycemia | Monitor | 7 |
| Methylprednisolone | Levofloxacin | H02 | J01 | PD-risk of tendon rupture | Moderate | 5 |
| Quetiapine | Ciprofloxacin, Levofloxacin | N05 | J01 | PD-QT prolongation | Monitor | 5 |
| Sertraline | N06 | Monitor | 2 | |||
| Risperidone | N05 | Monitor | 2 | |||
| Ramipril | Metformin | C09 | A10 | PD-hypoglycemia | Moderate | 3 |
| Salbutamol | Levofloxacin | R03 | J01 | PD-QT prolongation | Monitor | 6 |
| Quetiapine | N05 | Monitor | 3 | |||
| Escitalopram Fluoxetine | N06 | Monitor | 2 | |||
| Monitor | 2 | |||||
| Bisoprolol | C07 | PD-acute bronchospasm | Moderate | 3 | ||
| Spironlactone | KCl | C03 | A12 | PD-hyperkalemia | Monitor | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanakis, M.; Ioannou, P.; Tzalis, S.; Papakosta, V.; Patelarou, E.; Tzanakis, N.; Patelarou, A.; Kofteridis, D.P. Drug-Drug Interactions among Patients Hospitalized with COVID-19 in Greece. J. Clin. Med. 2022, 11, 7172. https://doi.org/10.3390/jcm11237172
Spanakis M, Ioannou P, Tzalis S, Papakosta V, Patelarou E, Tzanakis N, Patelarou A, Kofteridis DP. Drug-Drug Interactions among Patients Hospitalized with COVID-19 in Greece. Journal of Clinical Medicine. 2022; 11(23):7172. https://doi.org/10.3390/jcm11237172
Chicago/Turabian StyleSpanakis, Marios, Petros Ioannou, Sotiris Tzalis, Vasiliki Papakosta, Evridiki Patelarou, Nikos Tzanakis, Athina Patelarou, and Diamantis P. Kofteridis. 2022. "Drug-Drug Interactions among Patients Hospitalized with COVID-19 in Greece" Journal of Clinical Medicine 11, no. 23: 7172. https://doi.org/10.3390/jcm11237172
APA StyleSpanakis, M., Ioannou, P., Tzalis, S., Papakosta, V., Patelarou, E., Tzanakis, N., Patelarou, A., & Kofteridis, D. P. (2022). Drug-Drug Interactions among Patients Hospitalized with COVID-19 in Greece. Journal of Clinical Medicine, 11(23), 7172. https://doi.org/10.3390/jcm11237172