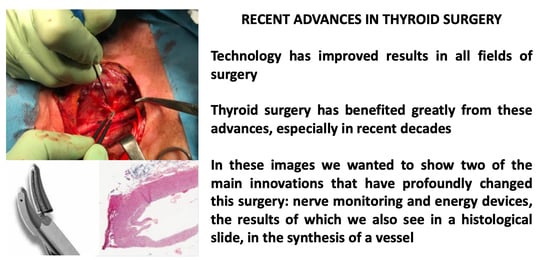
Editorial: Recent Advances in Thyroid Surgery
Funding
Conflicts of Interest
References
- Tröhler, U. Emil Theodor Kocher (1841–1917). J. R. Soc. Med. 2014, 107, 376–377. [Google Scholar] [CrossRef] [Green Version]
- Bergenfelz, A.; Jansson, S.; Kristoffersson, A.; Mårtensson, H.; Reihnér, E.; Wallin, G.; Lausen, I. Complications to thyroid surgery: Results as reported in a database from a multicenter audit comprising 3660 patients. Langenbeck’s Arch. Surg. 2008, 393, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Binenbaum, Y.; Cohen, J.T.; Gil, Z. Effectiveness of an oxidized cellulose patch hemostatic agent in thyroid surgery: A prospective, randomized, controlled study. J. Am. Coll. Surg. 2013, 217, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Testini, M.; Marzaioli, R.; Lissidini, G.; Lippolis, A.; Logoluso, F.; Gurrado, A.; Lardo, D.; Poli, E.; Piccinni, G. The effectiveness of FloSeal matrix hemostatic agent in thyroid surgery: A prospective, randomized, control study. Langenbeck’s Arch. Surg. 2009, 394, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Scerrino, G.; Paladino, N.C.; Di Paola, V.; Morfino, G.; Amodio, E.; Gulotta, G.; Bonventre, S. The use of haemostatic agents in thyroid surgery: Efficacy and further advantages. Collagen-Fibrinogen-Thrombin Patch (CFTP) versus Cellulose Gauze. Ann. Ital. Chir. 2013, 84, 545–550. [Google Scholar] [PubMed]
- Polychronidis, G.; Hüttner, F.J.; Contin, P.; Goossen, K.; Uhlmann, L.; Heidmann, M.; Knebel, P.; Diener, M.K.; Büchler, M.W.; Probst, P. Network meta-analysis of topical haemostatic agents in thyroid surgery. Br. J. Surg. 2018, 105, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Khadra, H.; Bakeer, M.; Hauch, A.; Hu, T.; Kandil, E. Hemostatic agent use in thyroid surgery: A meta-analysis. Gland Surg. 2018, 7 (Suppl. S1), S34–S41. [Google Scholar] [CrossRef]
- Canu, G.L.; Medas, F.; Podda, F.; Tatti, A.; Pisano, G.; Erdas, E.; Calò, P.G. Thyroidectomy with energy-based devices: Surgical outcomes and complications—Comparison between Harmonic Focus, LigaSure Small Jaw and Thunderbeat Open Fine Jaw. Gland Surg. 2020, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Shaari, A.L.; Spaulding, S.L.; Xing, M.H.; Yue, L.E.; Machadoa, R.A.; Moubayeda, S.P.; Mundia, N.; Chaia, R.L.; Urken, M.L. The anatomical basis for preserving the blood supply to the parathyroids during thyroid surgery, and a review of current technologic advances. Am. J. Otolaryngol. Head Neck Med. Surg. 2022, 42, 103161. [Google Scholar] [CrossRef]
- Romano, G.; Scerrino, G.; Profita, G.; Amato, G.; Salamone, G.; Di Buono, G.; Lo Piccolo, C.; Sorce, V.; Agrusa, A.; Gulotta, G. Terminal or truncal ligation of the inferior thyroid artery during thyroidectomy? A prospective randomized trial. Int. J. Surg. 2016, 28 (Suppl. S1), S13–S16. [Google Scholar] [CrossRef] [PubMed]
- Paladino, N.C.; Guérin, C.; Graziani, J.; Morange, I.; Loundou, A.; Taïeb, D.; Sebag, F. Predicting risk factors of postoperative hypocalcemia after total thyroidectomy: Is safe discharge without supplementation possible? A large cohort study. Langenbeck’s Arch. Surg. 2021, 406, 2425–2431. [Google Scholar] [CrossRef]
- Lu, W.; Chen, Q.; Zhang, P.; Su, A.; Zhu, J. Near-Infrared Autofluorescence Imaging in Thyroid Surgery: A Systematic Review and Meta-Analysis. J. Investig. Surg. 2022, 35, 1723–1732. [Google Scholar] [CrossRef]
- Schneider, R.; Randolph, G.W.; Barczynski, M.; Dionigi, G.; Wu, C.W.; Chiang, F.Y.; Machens, A.; Kamani, D.; Dralle, H. Continuous intraoperative neural monitoring of the recurrent nerves in thyroid surgery: A quantum leap in technology. Gland Surg. 2016, 5, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Dionigi, G.; Dralle, H.; Materazzi, G.; Kim, H.Y.; Miccoli, P. Happy 20th birthday to minimally invasive video-assisted thyroidectomy. J. Endocrinol. Investig. 2020, 43, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.P.; Raffaelli, M.; Traini, E.; De Crea, C.; Corsello, S.M.; Bellantone, R. Video-assisted minimally invasive parathyroidectomy: Benefits and long-term results. World J. Surg. 2009, 33, 2266–2281. [Google Scholar] [CrossRef]
- Scerrino, G.; Melfa, G.; Raspanti, C.; Rotolo, G.; Salamone, G.; Licari, L.; Fontana, T.; Tutino, R.; Porrello, C.; Gulotta, G.; et al. Minimally Invasive Video-Assisted Thyroidectomy: Analysis of Complications from a Systematic Review. Surg. Innov. 2019, 26, 381–387. [Google Scholar] [CrossRef]
- Graves, C.E.; Suh, I. The current status of remote access thyroidectomy in the United States. Surgery 2020, 168, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, R.; Egiddi, S.; Naciu, A.N.; Tabacco, G.; Leoncini, A.; Napoli, N.; Palermo, A.; Trimboli, P. Efficacy of radiofrequency and laser thermal ablation in solving thyroid nodule-related symptoms and cosmetic concerns. A systematic review and meta-analysis. Rev. Endocr. Metab. Dis. 2022, 23, 1051–1106. [Google Scholar] [CrossRef] [PubMed]
- Hamou, A.B.; Ghanassia, E.; Muller, A.; Ladsous, M.; Paladino, N.C.; Brunaud, L.; Leenhardt, L.; Russ, G. Section 8: Thermal ablation. In Annales d’Endocrinologie; Elsevier Masson: Paris, France, 2022. [Google Scholar] [CrossRef]
- Tennakoon, T.M.P.B.; Rushdhi, M.; Ranasinghe, A.D.C.U.; Dassanayake, R.S. Values of molecular markers in the differential diagnosis of thyroid abnormalities. J. Cancer Res. Clin. Oncol. 2017, 143, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Dolcetti, V.; Fresilli, D.; Del Gaudio, G.; Pacini, P.; Huang, P.; Camponovo, C.; Leoncini, A.; D’Andrea, V.; Pironi, D.; et al. The Role of CEUS in the Evaluation of Thyroid Cancer: From Diagnosis to Local Staging. J. Clin. Med. 2021, 10, 4559. [Google Scholar] [CrossRef]
- Cantisani, V.; De Silvestri, A.; Scotti, V.; Fresilli, D.; Tarsitano, M.G.; Polti, G.; Guiban, O.; Polito, E.; Pacini, P.; Durante, C.; et al. US-Elastography with Different Techniques for Thyroid Nodule Characterization: Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 845549. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Dolcetti, V.; Radzina, M.; Bellini, M.I.; Frezza, F.; Munir, K.; Grani, G.; Durante, C.; D’Andrea, V.; David, E.; et al. Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing? Cancers 2022, 14, 3357. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, A.M.; Lori, E.; Bellini, M.I.; Bolis, E.; Lozza, P.; Castellani, L.; Saibene, A.M.; Pipolo, C.; Fuccillo, E.; Rosso, C.; et al. Advanced Differentiated Thyroid Cancer: A Complex Condition Needing a Tailored Approach. Front. Oncol. 2022, 12, 954759. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scerrino, G.; Richiusa, P.; Graceffa, G.; Lori, E.; Sorrenti, S.; Paladino, N.C. Editorial: Recent Advances in Thyroid Surgery. J. Clin. Med. 2022, 11, 7233. https://doi.org/10.3390/jcm11237233
Scerrino G, Richiusa P, Graceffa G, Lori E, Sorrenti S, Paladino NC. Editorial: Recent Advances in Thyroid Surgery. Journal of Clinical Medicine. 2022; 11(23):7233. https://doi.org/10.3390/jcm11237233
Chicago/Turabian StyleScerrino, Gregorio, Pierina Richiusa, Giuseppa Graceffa, Eleonora Lori, Salvatore Sorrenti, and Nunzia Cinzia Paladino. 2022. "Editorial: Recent Advances in Thyroid Surgery" Journal of Clinical Medicine 11, no. 23: 7233. https://doi.org/10.3390/jcm11237233
APA StyleScerrino, G., Richiusa, P., Graceffa, G., Lori, E., Sorrenti, S., & Paladino, N. C. (2022). Editorial: Recent Advances in Thyroid Surgery. Journal of Clinical Medicine, 11(23), 7233. https://doi.org/10.3390/jcm11237233